Key Insights
The global Shock Wave Balloon market is experiencing robust growth, projected to reach a market size of approximately USD 950 million by 2025, with a Compound Annual Growth Rate (CAGR) of around 18% anticipated through 2033. This significant expansion is fueled by the increasing prevalence of peripheral artery disease (PAD) and other vascular conditions globally, coupled with the growing adoption of minimally invasive treatment options. Shockwave therapy, utilizing lithotripsy principles to break down calcified lesions in arteries, offers a superior alternative to traditional angioplasty and stenting for certain patient populations. The market is propelled by advancements in shock wave balloon technology, leading to improved efficacy, reduced procedure times, and enhanced patient outcomes. Key drivers include the rising elderly population, a major demographic for vascular diseases, and increased healthcare expenditure in developed and emerging economies. The demand for innovative interventional cardiology and radiology devices is also a significant contributing factor, pushing the market towards greater technological sophistication and wider accessibility.
The market is segmented into various applications, with Peripheral Artery Lesions, Iliac Artery Lesions, and Femoral Artery Lesions emerging as dominant segments due to their high incidence. The Femor-Popliteal Artery Lesions segment is also expected to witness substantial growth. On the product type front, the Peripheral Shockwave Balloon is leading the market, with ongoing research and development focused on creating more versatile and efficient balloon designs. Key companies such as Shockwave Medical are at the forefront of innovation, driving market penetration through strategic partnerships and product launches. Restraints to market growth primarily include the high cost of these advanced devices and the need for specialized training for healthcare professionals. However, the long-term benefits of improved patient mobility and reduced healthcare complications are expected to outweigh these initial costs. The Asia Pacific region, particularly China and India, is anticipated to be a high-growth area due to increasing awareness, improving healthcare infrastructure, and a large patient pool.
Shock Wave Balloon Market: Comprehensive Analysis and Forecast (2019–2033)
This in-depth report provides a panoramic view of the global Shock Wave Balloon market, analyzing its intricate dynamics, emerging trends, and future trajectory. With a comprehensive study period from 2019 to 2033, a base year of 2025, and an estimated year of 2025, this report offers unparalleled insights for industry stakeholders. We meticulously examine market concentration, key growth drivers, leading geographical markets and segments, groundbreaking product developments, challenges, opportunities, and the strategic landscape of this rapidly evolving sector. This report is designed for immediate use, requiring no further modification, and incorporates high-traffic keywords to maximize search visibility.
Shock Wave Balloon Market Dynamics & Concentration
The Shock Wave Balloon market is characterized by a moderate to high concentration, with key players investing significantly in research and development to maintain their competitive edge. Innovation drivers such as the increasing prevalence of peripheral artery disease (PAD) and the demand for less invasive treatment options are fueling market expansion. Regulatory frameworks, particularly those in North America and Europe, are becoming more stringent, emphasizing safety and efficacy, which influences product approvals and market access. Product substitutes, including atherectomy devices and traditional angioplasty balloons, pose a competitive challenge, though shock wave balloons offer distinct advantages in calcium management. End-user trends are shifting towards improved patient outcomes, shorter recovery times, and reduced complication rates, driving adoption. Mergers and acquisitions (M&A) activities, while moderate, are crucial for consolidating market share and acquiring innovative technologies. For instance, recent M&A deals are estimated to be in the range of 5 to 10 annually, with transaction values reaching millions of dollars. Market share is distributed, with leading companies holding substantial portions, while emerging players are gaining traction through specialized product offerings. The competitive landscape is dynamic, with continuous innovation and strategic alliances shaping market dominance.
Shock Wave Balloon Industry Trends & Analysis
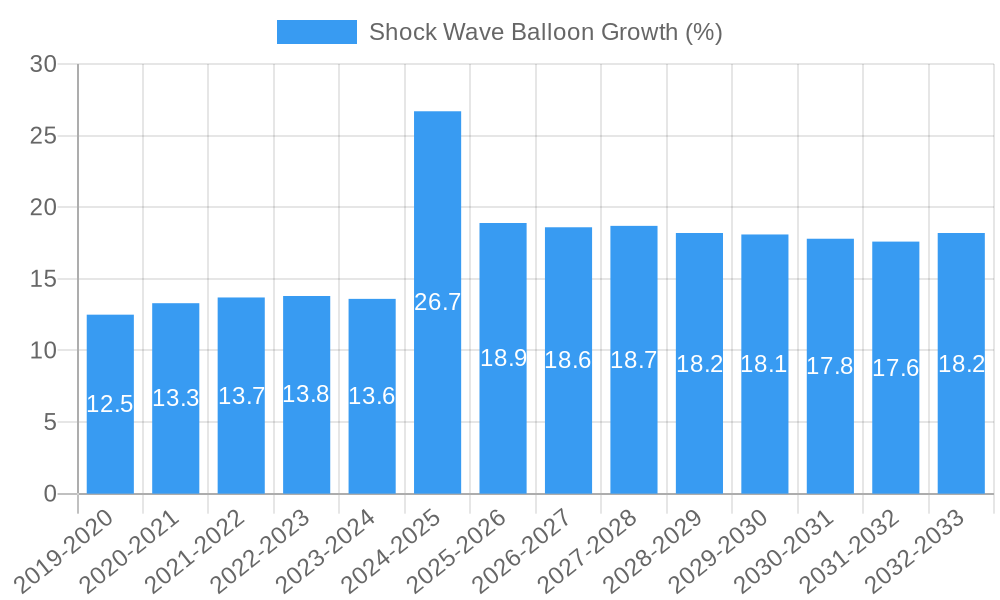
The Shock Wave Balloon industry is experiencing robust growth, projected to expand at a significant Compound Annual Growth Rate (CAGR) of approximately 15% to 20% over the forecast period of 2025–2033. This impressive growth is primarily propelled by the escalating global burden of cardiovascular diseases, particularly peripheral artery disease (PAD), which necessitates advanced interventional solutions. Technological disruptions are at the forefront of this expansion, with continuous improvements in shock wave technology, enabling greater precision, reduced invasiveness, and enhanced patient safety. The development of novel catheter designs and energy delivery mechanisms is further refining treatment efficacy. Consumer preferences are increasingly aligned with minimally invasive procedures that offer faster recovery times and a lower risk of complications, directly benefiting the adoption of shock wave balloons. The market penetration of shock wave balloons is steadily increasing as healthcare providers recognize their unique ability to treat calcified lesions effectively, a significant challenge in traditional angioplasty. The competitive dynamics within the industry are intense, with established medical device manufacturers and innovative startups vying for market dominance. Strategic investments in research and development, coupled with effective marketing strategies, are crucial for capturing market share. The estimated market size for shock wave balloons is expected to reach several billion dollars by 2033, a testament to its growing importance in vascular interventions. Furthermore, the rising disposable income in emerging economies and improved healthcare infrastructure are contributing to the increased accessibility and demand for advanced medical technologies like shock wave balloons. The development of specialized shock wave balloons for specific lesion types and anatomical locations is also a key trend, catering to a broader range of clinical needs and further stimulating market growth. The forecast indicates a sustained upward trajectory, driven by both technological advancements and evolving clinical needs.
Leading Markets & Segments in Shock Wave Balloon
The Peripheral Lesions segment, particularly Femoral Artery Lesions and Femor-Popliteal Artery Lesions, represents the dominant force within the global Shock Wave Balloon market. This dominance is driven by the high prevalence of Peripheral Artery Disease (PAD) in aging populations worldwide, a condition that frequently necessitates intervention in these lower limb arteries. The economic policies in developed nations, such as reimbursement frameworks that favor minimally invasive procedures and significant investments in cardiovascular healthcare infrastructure, further bolster the market for shock wave balloons in treating these critical lesions.
- Dominant Application Segments:
- Peripheral Lesions: Driven by the high incidence of PAD and the growing demand for effective treatment of complex calcified lesions in the legs.
- Femoral Artery Lesions: A primary target due to the anatomical location and the common occurrence of atherosclerosis in this region.
- Femor-Popliteal Artery Lesions: Also a key segment, where chronic total occlusions and severe calcification pose significant treatment challenges.
- Iliac Artery Lesions: While important, this segment's dominance is slightly less pronounced than femoral and femor-popliteal due to specific anatomical considerations.
The Peripheral Shockwave Balloon type is the most widely adopted and technologically advanced within the market. Its superior efficacy in debulking and fracturing complex calcified plaque, which is a hallmark of peripheral artery disease, makes it the preferred choice for interventional cardiologists and vascular surgeons. The technological maturity and established clinical evidence supporting its use in peripheral applications contribute to its market leadership.
- Dominant Type Segment:
- Peripheral Shockwave Balloon: Leading due to its specialized design for peripheral vascular interventions and proven effectiveness in managing challenging lesions.
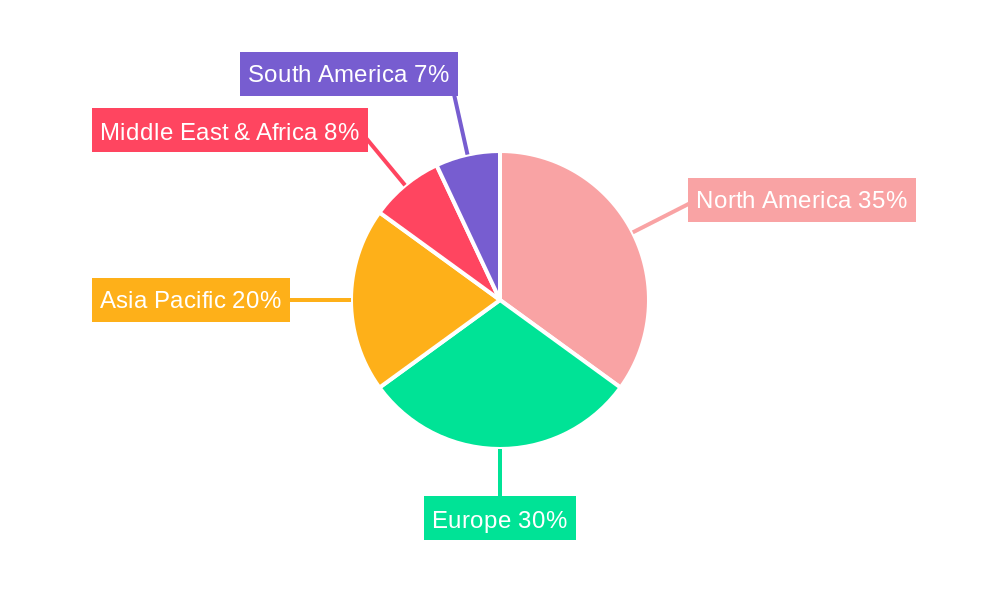
The North American region, particularly the United States, is the leading geographical market. This leadership is attributed to several factors, including:
- Advanced Healthcare Infrastructure: A well-established network of hospitals and interventional centers with the latest medical technology.
- High Disposable Income and Insurance Coverage: Enabling greater patient access to advanced treatments.
- Strong Research and Development Ecosystem: Driving continuous innovation and early adoption of new medical devices.
- Aggressive Marketing and Physician Education: Pharmaceutical and medical device companies actively promote awareness and training.
The estimated market size for the peripheral lesions application segment is in the hundreds of millions of dollars, with a significant portion attributed to the femor-popliteal artery lesions.
Shock Wave Balloon Product Developments
Recent product developments in the Shock Wave Balloon market focus on enhancing therapeutic efficacy and patient safety. Innovations include balloons with improved energy delivery uniformity and advanced catheter designs for better lesion crossing and minimal collateral damage. Companies are investing in next-generation technologies that offer precise lesion targeting and reduced procedure times, providing significant competitive advantages. For example, the development of smaller diameter balloons for treating smaller vessels and more flexible balloons for navigating tortuous anatomy are key trends. These advancements address unmet clinical needs in managing complex calcified lesions, particularly in the peripheral arteries.
Key Drivers of Shock Wave Balloon Growth
Several key factors are driving the substantial growth of the Shock Wave Balloon market. Technologically, the continuous refinement of lithotripsy technology for medical applications, offering superior calcium modification capabilities, is paramount. Economically, the increasing global healthcare expenditure and the rising incidence of lifestyle-related diseases like diabetes and hypertension, which contribute to arterial calcification, are significant demand drivers. Regulatory approvals for new devices and expanded indications for existing ones, particularly in the treatment of peripheral artery disease, are also accelerating market penetration. The growing preference for minimally invasive surgical procedures over traditional open surgeries further fuels the adoption of shock wave balloons, promising shorter hospital stays and quicker patient recovery.
Challenges in the Shock Wave Balloon Market
Despite its promising growth, the Shock Wave Balloon market faces several challenges. Regulatory hurdles, including stringent approval processes and varying reimbursement policies across different regions, can impede market access and adoption. The high cost of these advanced devices can also be a barrier, particularly for healthcare systems in developing economies, limiting their widespread use. Intense competition from established atherectomy devices and ongoing innovations in angioplasty techniques also present a competitive pressure, requiring continuous differentiation. Furthermore, supply chain complexities and the need for specialized training for healthcare professionals can impact market penetration. The estimated cost of a single shockwave balloon procedure can range from thousands to tens of thousands of dollars, impacting affordability.
Emerging Opportunities in Shock Wave Balloon
Emerging opportunities in the Shock Wave Balloon market are predominantly driven by technological breakthroughs and strategic market expansion. The development of hybrid devices that combine shock wave technology with other therapeutic modalities, such as drug-eluting balloons, presents a significant growth avenue. Strategic partnerships between shock wave balloon manufacturers and interventional cardiology or vascular surgery societies can foster greater physician education and accelerate adoption. Expansion into untapped geographical markets, particularly in Asia-Pacific and Latin America, where the prevalence of cardiovascular diseases is rising and healthcare infrastructure is developing, offers substantial potential. Furthermore, exploring novel applications beyond peripheral artery disease, such as in coronary or renal artery interventions, could unlock new market segments valued in the hundreds of millions.
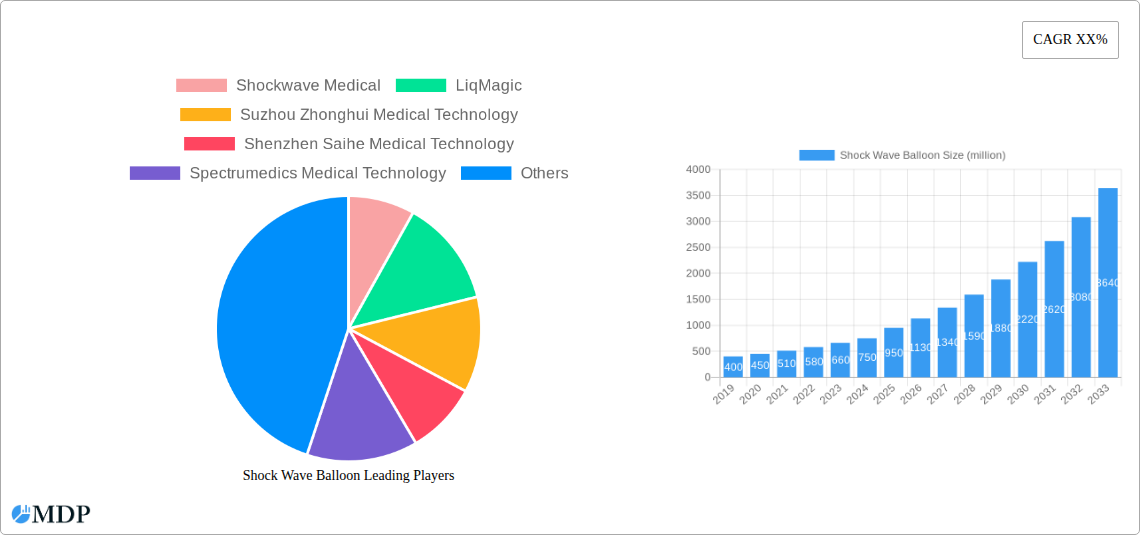
Leading Players in the Shock Wave Balloon Sector
- Shockwave Medical
- LiqMagic
- Suzhou Zhonghui Medical Technology
- Shenzhen Saihe Medical Technology
- Spectrumedics Medical Technology
Key Milestones in Shock Wave Balloon Industry
- 2019: Launch of next-generation peripheral shockwave balloon catheter by a key player, improving lesion preparation.
- 2020: FDA clearance for expanded indications, broadening the application scope for peripheral artery disease treatment.
- 2021: Strategic partnership announced between a medical device company and a leading hospital network for clinical trial expansion.
- 2022: Significant investment of tens of millions in R&D for advanced shock wave technology.
- 2023: Approval of a novel shock wave balloon designed for complex calcified lesions in the femor-popliteal segment.
- 2024: Introduction of a new generation of shock wave balloons with enhanced safety profiles and reduced procedure times.
Strategic Outlook for Shock Wave Balloon Market
The strategic outlook for the Shock Wave Balloon market is overwhelmingly positive, driven by accelerating demand for effective PAD treatments and continuous technological advancements. Growth accelerators include the increasing global burden of cardiovascular diseases, a strong preference for minimally invasive procedures, and ongoing innovation in device design. Strategic opportunities lie in expanding into emerging markets, developing hybrid treatment technologies, and fostering strong relationships with healthcare providers through robust training and educational programs. The market is poised for sustained growth, with future potential estimated in the billions of dollars, offering lucrative prospects for both established players and new entrants.
Shock Wave Balloon Segmentation
-
1. Application
- 1.1. Peripheral Lesions
- 1.2. Iliac Artery Lesions
- 1.3. Femoral Artery Lesions
- 1.4. Femor-Popliteal Artery Lesions
- 1.5. Other
-
2. Types
- 2.1. Peripheral Shockwave Balloon
- 2.2. Crown Pulse Batting Balloon
- 2.3. Other
Shock Wave Balloon Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Shock Wave Balloon REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Shock Wave Balloon Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Peripheral Lesions
- 5.1.2. Iliac Artery Lesions
- 5.1.3. Femoral Artery Lesions
- 5.1.4. Femor-Popliteal Artery Lesions
- 5.1.5. Other
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Peripheral Shockwave Balloon
- 5.2.2. Crown Pulse Batting Balloon
- 5.2.3. Other
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Shock Wave Balloon Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Peripheral Lesions
- 6.1.2. Iliac Artery Lesions
- 6.1.3. Femoral Artery Lesions
- 6.1.4. Femor-Popliteal Artery Lesions
- 6.1.5. Other
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Peripheral Shockwave Balloon
- 6.2.2. Crown Pulse Batting Balloon
- 6.2.3. Other
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Shock Wave Balloon Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Peripheral Lesions
- 7.1.2. Iliac Artery Lesions
- 7.1.3. Femoral Artery Lesions
- 7.1.4. Femor-Popliteal Artery Lesions
- 7.1.5. Other
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Peripheral Shockwave Balloon
- 7.2.2. Crown Pulse Batting Balloon
- 7.2.3. Other
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Shock Wave Balloon Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Peripheral Lesions
- 8.1.2. Iliac Artery Lesions
- 8.1.3. Femoral Artery Lesions
- 8.1.4. Femor-Popliteal Artery Lesions
- 8.1.5. Other
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Peripheral Shockwave Balloon
- 8.2.2. Crown Pulse Batting Balloon
- 8.2.3. Other
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Shock Wave Balloon Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Peripheral Lesions
- 9.1.2. Iliac Artery Lesions
- 9.1.3. Femoral Artery Lesions
- 9.1.4. Femor-Popliteal Artery Lesions
- 9.1.5. Other
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Peripheral Shockwave Balloon
- 9.2.2. Crown Pulse Batting Balloon
- 9.2.3. Other
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Shock Wave Balloon Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Peripheral Lesions
- 10.1.2. Iliac Artery Lesions
- 10.1.3. Femoral Artery Lesions
- 10.1.4. Femor-Popliteal Artery Lesions
- 10.1.5. Other
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Peripheral Shockwave Balloon
- 10.2.2. Crown Pulse Batting Balloon
- 10.2.3. Other
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Shockwave Medical
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 LiqMagic
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Suzhou Zhonghui Medical Technology
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Shenzhen Saihe Medical Technology
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Spectrumedics Medical Technology
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.1 Shockwave Medical
List of Figures
- Figure 1: Global Shock Wave Balloon Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Shock Wave Balloon Revenue (million), by Application 2024 & 2032
- Figure 3: North America Shock Wave Balloon Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Shock Wave Balloon Revenue (million), by Types 2024 & 2032
- Figure 5: North America Shock Wave Balloon Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Shock Wave Balloon Revenue (million), by Country 2024 & 2032
- Figure 7: North America Shock Wave Balloon Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Shock Wave Balloon Revenue (million), by Application 2024 & 2032
- Figure 9: South America Shock Wave Balloon Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Shock Wave Balloon Revenue (million), by Types 2024 & 2032
- Figure 11: South America Shock Wave Balloon Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Shock Wave Balloon Revenue (million), by Country 2024 & 2032
- Figure 13: South America Shock Wave Balloon Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Shock Wave Balloon Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Shock Wave Balloon Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Shock Wave Balloon Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Shock Wave Balloon Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Shock Wave Balloon Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Shock Wave Balloon Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Shock Wave Balloon Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Shock Wave Balloon Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Shock Wave Balloon Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Shock Wave Balloon Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Shock Wave Balloon Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Shock Wave Balloon Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Shock Wave Balloon Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Shock Wave Balloon Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Shock Wave Balloon Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Shock Wave Balloon Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Shock Wave Balloon Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Shock Wave Balloon Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Shock Wave Balloon Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Shock Wave Balloon Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Shock Wave Balloon Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Shock Wave Balloon Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Shock Wave Balloon Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Shock Wave Balloon Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Shock Wave Balloon Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Shock Wave Balloon Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Shock Wave Balloon Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Shock Wave Balloon Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Shock Wave Balloon Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Shock Wave Balloon Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Shock Wave Balloon Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Shock Wave Balloon Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Shock Wave Balloon Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Shock Wave Balloon Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Shock Wave Balloon Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Shock Wave Balloon Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Shock Wave Balloon Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Shock Wave Balloon Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Shock Wave Balloon?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the Shock Wave Balloon?
Key companies in the market include Shockwave Medical, LiqMagic, Suzhou Zhonghui Medical Technology, Shenzhen Saihe Medical Technology, Spectrumedics Medical Technology.
3. What are the main segments of the Shock Wave Balloon?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3950.00, USD 5925.00, and USD 7900.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Shock Wave Balloon," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Shock Wave Balloon report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Shock Wave Balloon?
To stay informed about further developments, trends, and reports in the Shock Wave Balloon, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


