Key Insights
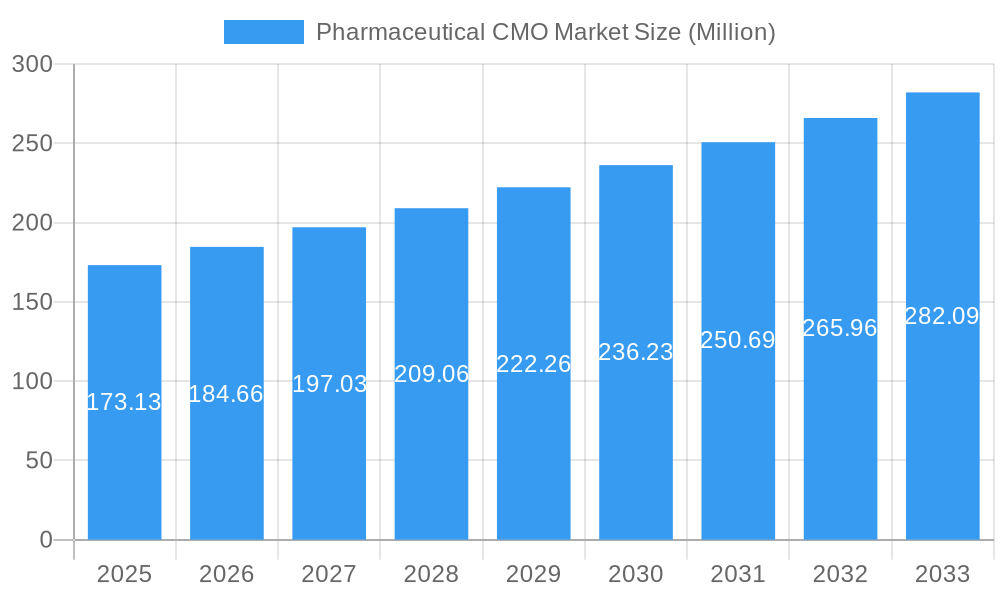
The Pharmaceutical Contract Manufacturing Organization (CMO) market, valued at $173.13 million in 2025, is projected to experience robust growth, driven by a compound annual growth rate (CAGR) of 6.53% from 2025 to 2033. This expansion is fueled by several key factors. The increasing demand for specialized pharmaceutical products, coupled with the rising complexity of drug development and manufacturing processes, is prompting pharmaceutical companies to outsource these activities to CMOs. This allows them to focus on core competencies like research and development while leveraging the expertise and economies of scale offered by specialized contract manufacturers. Further driving market growth is the increasing prevalence of outsourcing among smaller pharmaceutical firms lacking the resources to invest in extensive in-house manufacturing capabilities. The market is segmented by service type, including Active Pharmaceutical Ingredient (API) manufacturing, high-potency API (HPAPI) and finished dosage formulation (FDF) development and manufacturing, solid and injectable dose formulations, and secondary packaging. Geographical expansion, particularly in emerging markets in Asia, is also contributing significantly to overall market growth. Competition within the market is intense, with key players like Lonza Group, Catalent Inc., and Thermo Fisher Scientific continuously investing in advanced technologies and expanding their service offerings to maintain a competitive edge.

Pharmaceutical CMO Market Market Size (In Million)
The market's growth trajectory is likely to be influenced by several trends. The rising adoption of advanced technologies such as continuous manufacturing and digitalization within pharmaceutical production processes will improve efficiency and reduce manufacturing costs, further boosting the appeal of CMOs. Stringent regulatory requirements and increasing quality control standards will necessitate substantial investment in compliance infrastructure by CMOs, potentially influencing pricing and market consolidation. Despite these challenges, the overall outlook remains positive, indicating strong future prospects for the pharmaceutical CMO market. The considerable investment in research and development of novel drug therapies and the ongoing need for efficient and cost-effective manufacturing processes will continue to be major drivers of sustained growth throughout the forecast period.
Pharmaceutical CMO Market Company Market Share
Pharmaceutical CMO Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the Pharmaceutical Contract Manufacturing Organization (CMO) market, offering invaluable insights for industry stakeholders, investors, and strategic decision-makers. With a detailed examination of market dynamics, leading players, and future trends, this report is an essential resource for navigating this rapidly evolving landscape. The report covers the period 2019-2033, with a base year of 2025 and a forecast period from 2025-2033. Market values are expressed in Millions.
Pharmaceutical CMO Market Market Dynamics & Concentration
The Pharmaceutical CMO market is characterized by a moderately concentrated landscape, with a handful of large players holding significant market share. Market concentration is influenced by factors such as economies of scale, technological capabilities, and regulatory compliance. Innovation is a crucial driver, particularly in areas like advanced drug delivery systems and High Potency API (HPAPI) manufacturing. Stringent regulatory frameworks, including GMP (Good Manufacturing Practice) compliance, pose significant barriers to entry but simultaneously ensure high quality standards. The market witnesses continuous product substitution as newer technologies and formulations emerge, requiring CMOs to adapt and upgrade their capabilities. End-user trends such as the increasing demand for biologics and personalized medicine directly impact the services required from CMOs. Mergers and acquisitions (M&A) activities are frequent, with larger players seeking to expand their service portfolios and geographic reach. In 2024, approximately xx M&A deals were recorded, resulting in a market share shift of approximately xx%. The top 5 players collectively held approximately xx% of the global market share in 2024.
Pharmaceutical CMO Market Industry Trends & Analysis
The Pharmaceutical CMO market is experiencing robust growth, driven by several key factors. The increasing outsourcing trend by pharmaceutical companies, particularly small and mid-sized enterprises (SMEs), seeking to reduce operational costs and focus on R&D, is a significant catalyst. Technological advancements, such as the adoption of automation and digitalization in manufacturing processes, are enhancing efficiency and productivity. Consumer preferences for innovative drug delivery systems, such as injectables and personalized medicines, are driving demand for specialized CMO services. The competitive landscape is highly dynamic, with companies constantly striving to enhance their service offerings and technological capabilities to gain a competitive edge. The Compound Annual Growth Rate (CAGR) for the period 2025-2033 is projected at xx%, indicating strong market expansion. Market penetration of advanced technologies like AI and machine learning is expected to reach xx% by 2033.
Leading Markets & Segments in Pharmaceutical CMO Market
The Pharmaceutical Contract Manufacturing Organization (CMO) market is a dynamic global landscape, with North America currently leading in market share. This dominance is attributed to substantial investments in research and development, the significant presence of major pharmaceutical corporations, and a mature, well-defined regulatory environment. Europe and the Asia Pacific regions are also key players, demonstrating robust growth trajectories. The Asia Pacific, in particular, is anticipated to experience accelerated expansion, driven by the rise of emerging economies and increasing manufacturing capabilities.
A granular analysis of market segments by service type reveals that Active Pharmaceutical Ingredient (API) Manufacturing and Finished Dosage Formulation (FDF) Development and Manufacturing represent the largest and most influential segments. The specific catalysts driving growth within these segments are multifaceted:
- Active Pharmaceutical Ingredient (API) Manufacturing: This segment's expansion is propelled by a sustained high demand for generic medications, ongoing efforts to optimize API manufacturing costs, and a strategic trend among large pharmaceutical enterprises to increasingly outsource API production.
- High Potency API (HPAPI) Manufacturing: The surging demand for oncology drugs and other highly potent therapeutic agents, coupled with the imperative for stringent safety protocols and specialized handling expertise, is the primary driver for this specialized segment.
- Finished Dosage Formulation (FDF): The growing need for personalized and highly specialized medications, alongside the pressure to expedite turnaround times in FDF development and manufacturing processes, fuels growth in this area.
- Solid Dose Formulation (Tablets, Capsules, Powders): Leveraging well-established manufacturing technologies, this segment continues to command a significant portion of the market due to its widespread application and cost-effectiveness.
- Injectable Dose Formulation: The continuous advancements in biologics and the increasing sophistication of specialized drug delivery systems are key drivers behind the escalating demand for injectable formulations.
- Secondary Packaging: This segment's growth is intrinsically linked to the performance of the primary formulation segments, with a strong emphasis on cost optimization and the pursuit of enhanced operational efficiency.
Over the next decade, these key segments are projected to exhibit impressive Compound Annual Growth Rates (CAGRs): approximately xx% for API Manufacturing, around xx% for HPAPI Manufacturing, and a notable xx% for FDF. Solid Dose and Injectable formulations are also expected to experience substantial growth, contributing significantly to the overall market expansion. This growth is further underpinned by supportive economic policies, well-developed logistical and manufacturing infrastructure, and the availability of a highly skilled workforce.
Pharmaceutical CMO Market Product Developments
Recent advancements in the Pharmaceutical CMO market are heavily focused on the integration of cutting-edge technologies to enhance efficiency and product quality. A prime example is the adoption of continuous manufacturing processes, which are revolutionizing production by significantly reducing costs, minimizing waste, and improving overall output consistency. Alongside this, there is a marked acceleration in innovations related to advanced drug delivery systems. These include sophisticated methods for targeted drug delivery, ensuring therapeutic agents reach their intended sites of action more effectively, and formulations designed to significantly enhance drug bioavailability, leading to improved patient outcomes. These developments empower CMOs to offer distinct competitive advantages, enabling them to deliver superior product quality, drastically reduce development and manufacturing timelines, and provide bespoke, innovative solutions precisely tailored to the unique requirements of their pharmaceutical partners. Furthermore, the increasing embrace of digital technologies, such as Artificial Intelligence (AI) and Machine Learning (ML), is proving transformative, optimizing production workflows, predicting and mitigating potential issues, and ultimately enhancing overall yield and efficacy.
Key Drivers of Pharmaceutical CMO Market Growth
The robust expansion of the Pharmaceutical CMO market is propelled by a confluence of strategic and operational factors:
- Increased Outsourcing by Pharmaceutical Companies: A growing number of pharmaceutical firms are strategically outsourcing manufacturing activities to CMOs. This allows them to concentrate valuable resources on core competencies such as research and development (R&D) and commercialization, while simultaneously reducing operational overheads and gaining access to specialized expertise and advanced manufacturing capabilities.
- Technological Advancements: The relentless pace of technological innovation, including the widespread adoption of automation, digitalization of processes, and the implementation of continuous manufacturing techniques, is significantly boosting efficiency, ensuring higher product quality, and fostering greater flexibility in production.
- Stringent Regulatory Requirements: The evolving and increasingly demanding global regulatory landscape, with its emphasis on Good Manufacturing Practices (GMP) and product safety, necessitates high-quality, compliant manufacturing services, which CMOs are well-positioned to provide.
- Growing Demand for Specialized Formulations: The rise of complex therapeutics such as biologics, the increasing focus on personalized medicine, and the development of sophisticated advanced drug delivery systems are creating a strong demand for specialized manufacturing expertise that many CMOs possess.
- Expansion of the Global Pharmaceutical Market: Underlying these trends is the steady growth of the global pharmaceutical market itself, fueled by an aging worldwide population, the escalating prevalence of chronic diseases, and rising healthcare expenditures in both developed and emerging economies.
Challenges in the Pharmaceutical CMO Market
The Pharmaceutical CMO market faces several challenges, including:
- Stringent regulatory compliance: Meeting GMP standards and navigating complex regulatory landscapes poses significant challenges. Non-compliance can lead to significant financial penalties and reputational damage. Estimates suggest that non-compliance costs companies an average of xx Million annually.
- Supply chain disruptions: Raw material shortages and logistical challenges can impact production timelines and costs. These disruptions cost companies an estimated xx Million annually.
- Intense competition: The market is characterized by intense competition among established players and new entrants, requiring constant innovation and cost optimization. Competitive pressures are estimated to lower profit margins by xx% annually.
Emerging Opportunities in Pharmaceutical CMO Market
The Pharmaceutical CMO sector is ripe with compelling opportunities poised to shape its future trajectory. The integration of transformative technologies like Artificial Intelligence (AI) and advanced data analytics presents substantial potential for optimizing manufacturing processes, predicting market trends, and accelerating product development cycles. Furthermore, the formation of strategic alliances and partnerships between CMOs and pharmaceutical companies is becoming increasingly vital. These collaborations foster seamless technology transfer, enhance operational synergy, and accelerate the path from discovery to market. Significant growth prospects also lie in market expansion into emerging economies, particularly in regions like Asia and Africa. CMOs that can adapt to local regulatory frameworks, address specific market needs, and establish robust supply chains in these territories stand to gain considerable market share. These strategic initiatives and market penetrations are projected to contribute an estimated xx% increase to the market size within the next five years.
Leading Players in the Pharmaceutical CMO Market Sector
- Famar SA
- Lonza Group
- Tesa Labtec GmbH (Tesa SE)
- ARX LL
- Patheon Inc (Thermo Fisher Scientific Inc)
- Pfizer CentreSource (Pfizer Inc)
- Tapemark
- Aenova Holdings GmbH
- Catalent Inc
- Boehringer Ingelheim Group
- Recipharm AB
- Baxter Biopharma Solutions (Baxter International Inc)
- Jubilant Biosys Ltd (Jubilant Pharmova Ltd)
Key Milestones in Pharmaceutical CMO Market Industry
- January 2023: Catalent's agreement with Ethicann Pharmaceuticals for CBD/THC drug development using Zydis technology signifies the growing interest in cannabinoid-based therapies and the role of CMOs in supporting clinical trials.
- March 2023: Biose Industria's new facility in Boston strengthens its US market presence and highlights the increasing demand for Live Biotech Process Development and Production (LBP) services.
- July 2023: Recipharm's new analytical laboratory in Bangalore expands its global testing capabilities, emphasizing the importance of quality control and regulatory compliance in the CMO sector.
Strategic Outlook for Pharmaceutical CMO Market Market
The outlook for the Pharmaceutical CMO market is exceptionally promising, fueled by sustained trends of pharmaceutical companies prioritizing core R&D and outsourcing manufacturing. The relentless march of technological innovation, coupled with an ever-growing demand for specialized and complex pharmaceutical services, will continue to be key growth catalysts. To thrive and lead in this competitive landscape, CMOs must focus on cultivating robust strategic partnerships, making judicious investments in cutting-edge technologies, and strategically expanding their geographical footprint into new and growing markets. A steadfast commitment to delivering exceptionally high-quality, compliant services, underpinned by a culture of continuous innovation and operational efficiency, will be the defining characteristics of market leaders. The industry is well-positioned for substantial and sustained growth, driven by ongoing advancements in pharmaceutical R&D and the persistent need for efficient, cost-effective, and reliable manufacturing solutions.
Pharmaceutical CMO Market Segmentation
-
1. Service Type
-
1.1. Active P
- 1.1.1. Small Molecule
- 1.1.2. Large Molecule
- 1.1.3. High Potency API (HPAPI)
-
1.2. Finished
-
1.2.1. Solid Dose Formulation
- 1.2.1.1. Tablets
- 1.2.1.2. Other Types (Capsules, Powders, etc.)
- 1.2.2. Liquid Dose Formulation
- 1.2.3. Injectable Dose Formulation
-
1.2.1. Solid Dose Formulation
- 1.3. Secondary Packaging
-
1.1. Active P
Pharmaceutical CMO Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
-
2. Europe
- 2.1. United Kingdom
- 2.2. Germany
- 2.3. France
- 2.4. Italy
- 2.5. Spain
-
3. Asia
- 3.1. China
- 3.2. India
- 3.3. Japan
- 3.4. Australia
- 4. Australia and New Zealand
-
5. Latin America
- 5.1. Brazil
- 5.2. Mexico
- 5.3. Argentina
-
6. Middle East and Africa
- 6.1. United Arab Emirates
- 6.2. Saudi Arabia
- 6.3. South Africa

Pharmaceutical CMO Market Regional Market Share
Geographic Coverage of Pharmaceutical CMO Market
Pharmaceutical CMO Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.53% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Outsourcing Volume by Pharmaceutical Companies; Increasing Investment in Research and Development
- 3.3. Market Restrains
- 3.3.1. Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs
- 3.4. Market Trends
- 3.4.1. Active Pharmaceutical Ingredient (API) and Intermediates are Expected to Witness Robust Demand
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Service Type
- 5.1.1. Active P
- 5.1.1.1. Small Molecule
- 5.1.1.2. Large Molecule
- 5.1.1.3. High Potency API (HPAPI)
- 5.1.2. Finished
- 5.1.2.1. Solid Dose Formulation
- 5.1.2.1.1. Tablets
- 5.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 5.1.2.2. Liquid Dose Formulation
- 5.1.2.3. Injectable Dose Formulation
- 5.1.2.1. Solid Dose Formulation
- 5.1.3. Secondary Packaging
- 5.1.1. Active P
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. North America
- 5.2.2. Europe
- 5.2.3. Asia
- 5.2.4. Australia and New Zealand
- 5.2.5. Latin America
- 5.2.6. Middle East and Africa
- 5.1. Market Analysis, Insights and Forecast - by Service Type
- 6. North America Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Service Type
- 6.1.1. Active P
- 6.1.1.1. Small Molecule
- 6.1.1.2. Large Molecule
- 6.1.1.3. High Potency API (HPAPI)
- 6.1.2. Finished
- 6.1.2.1. Solid Dose Formulation
- 6.1.2.1.1. Tablets
- 6.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 6.1.2.2. Liquid Dose Formulation
- 6.1.2.3. Injectable Dose Formulation
- 6.1.2.1. Solid Dose Formulation
- 6.1.3. Secondary Packaging
- 6.1.1. Active P
- 6.1. Market Analysis, Insights and Forecast - by Service Type
- 7. Europe Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Service Type
- 7.1.1. Active P
- 7.1.1.1. Small Molecule
- 7.1.1.2. Large Molecule
- 7.1.1.3. High Potency API (HPAPI)
- 7.1.2. Finished
- 7.1.2.1. Solid Dose Formulation
- 7.1.2.1.1. Tablets
- 7.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 7.1.2.2. Liquid Dose Formulation
- 7.1.2.3. Injectable Dose Formulation
- 7.1.2.1. Solid Dose Formulation
- 7.1.3. Secondary Packaging
- 7.1.1. Active P
- 7.1. Market Analysis, Insights and Forecast - by Service Type
- 8. Asia Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Service Type
- 8.1.1. Active P
- 8.1.1.1. Small Molecule
- 8.1.1.2. Large Molecule
- 8.1.1.3. High Potency API (HPAPI)
- 8.1.2. Finished
- 8.1.2.1. Solid Dose Formulation
- 8.1.2.1.1. Tablets
- 8.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 8.1.2.2. Liquid Dose Formulation
- 8.1.2.3. Injectable Dose Formulation
- 8.1.2.1. Solid Dose Formulation
- 8.1.3. Secondary Packaging
- 8.1.1. Active P
- 8.1. Market Analysis, Insights and Forecast - by Service Type
- 9. Australia and New Zealand Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Service Type
- 9.1.1. Active P
- 9.1.1.1. Small Molecule
- 9.1.1.2. Large Molecule
- 9.1.1.3. High Potency API (HPAPI)
- 9.1.2. Finished
- 9.1.2.1. Solid Dose Formulation
- 9.1.2.1.1. Tablets
- 9.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 9.1.2.2. Liquid Dose Formulation
- 9.1.2.3. Injectable Dose Formulation
- 9.1.2.1. Solid Dose Formulation
- 9.1.3. Secondary Packaging
- 9.1.1. Active P
- 9.1. Market Analysis, Insights and Forecast - by Service Type
- 10. Latin America Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Service Type
- 10.1.1. Active P
- 10.1.1.1. Small Molecule
- 10.1.1.2. Large Molecule
- 10.1.1.3. High Potency API (HPAPI)
- 10.1.2. Finished
- 10.1.2.1. Solid Dose Formulation
- 10.1.2.1.1. Tablets
- 10.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 10.1.2.2. Liquid Dose Formulation
- 10.1.2.3. Injectable Dose Formulation
- 10.1.2.1. Solid Dose Formulation
- 10.1.3. Secondary Packaging
- 10.1.1. Active P
- 10.1. Market Analysis, Insights and Forecast - by Service Type
- 11. Middle East and Africa Pharmaceutical CMO Market Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Service Type
- 11.1.1. Active P
- 11.1.1.1. Small Molecule
- 11.1.1.2. Large Molecule
- 11.1.1.3. High Potency API (HPAPI)
- 11.1.2. Finished
- 11.1.2.1. Solid Dose Formulation
- 11.1.2.1.1. Tablets
- 11.1.2.1.2. Other Types (Capsules, Powders, etc.)
- 11.1.2.2. Liquid Dose Formulation
- 11.1.2.3. Injectable Dose Formulation
- 11.1.2.1. Solid Dose Formulation
- 11.1.3. Secondary Packaging
- 11.1.1. Active P
- 11.1. Market Analysis, Insights and Forecast - by Service Type
- 12. Competitive Analysis
- 12.1. Global Market Share Analysis 2025
- 12.2. Company Profiles
- 12.2.1 Famar SA
- 12.2.1.1. Overview
- 12.2.1.2. Products
- 12.2.1.3. SWOT Analysis
- 12.2.1.4. Recent Developments
- 12.2.1.5. Financials (Based on Availability)
- 12.2.2 Lonza Group
- 12.2.2.1. Overview
- 12.2.2.2. Products
- 12.2.2.3. SWOT Analysis
- 12.2.2.4. Recent Developments
- 12.2.2.5. Financials (Based on Availability)
- 12.2.3 Tesa Labtec GmbH (Tesa SE)
- 12.2.3.1. Overview
- 12.2.3.2. Products
- 12.2.3.3. SWOT Analysis
- 12.2.3.4. Recent Developments
- 12.2.3.5. Financials (Based on Availability)
- 12.2.4 ARX LL
- 12.2.4.1. Overview
- 12.2.4.2. Products
- 12.2.4.3. SWOT Analysis
- 12.2.4.4. Recent Developments
- 12.2.4.5. Financials (Based on Availability)
- 12.2.5 Patheon Inc (Thermo Fisher Scientific Inc )
- 12.2.5.1. Overview
- 12.2.5.2. Products
- 12.2.5.3. SWOT Analysis
- 12.2.5.4. Recent Developments
- 12.2.5.5. Financials (Based on Availability)
- 12.2.6 Pfizer CentreSource (Pfizer Inc )
- 12.2.6.1. Overview
- 12.2.6.2. Products
- 12.2.6.3. SWOT Analysis
- 12.2.6.4. Recent Developments
- 12.2.6.5. Financials (Based on Availability)
- 12.2.7 Tapemark
- 12.2.7.1. Overview
- 12.2.7.2. Products
- 12.2.7.3. SWOT Analysis
- 12.2.7.4. Recent Developments
- 12.2.7.5. Financials (Based on Availability)
- 12.2.8 Aenova Holdings GmbH
- 12.2.8.1. Overview
- 12.2.8.2. Products
- 12.2.8.3. SWOT Analysis
- 12.2.8.4. Recent Developments
- 12.2.8.5. Financials (Based on Availability)
- 12.2.9 Catalent Inc
- 12.2.9.1. Overview
- 12.2.9.2. Products
- 12.2.9.3. SWOT Analysis
- 12.2.9.4. Recent Developments
- 12.2.9.5. Financials (Based on Availability)
- 12.2.10 Boehringer Ingelheim Group
- 12.2.10.1. Overview
- 12.2.10.2. Products
- 12.2.10.3. SWOT Analysis
- 12.2.10.4. Recent Developments
- 12.2.10.5. Financials (Based on Availability)
- 12.2.11 Recipharm AB
- 12.2.11.1. Overview
- 12.2.11.2. Products
- 12.2.11.3. SWOT Analysis
- 12.2.11.4. Recent Developments
- 12.2.11.5. Financials (Based on Availability)
- 12.2.12 Baxter Biopharma Solutions (Baxter International Inc )
- 12.2.12.1. Overview
- 12.2.12.2. Products
- 12.2.12.3. SWOT Analysis
- 12.2.12.4. Recent Developments
- 12.2.12.5. Financials (Based on Availability)
- 12.2.13 Jubilant Biosys Ltd (Jubilant Pharmova Ltd)
- 12.2.13.1. Overview
- 12.2.13.2. Products
- 12.2.13.3. SWOT Analysis
- 12.2.13.4. Recent Developments
- 12.2.13.5. Financials (Based on Availability)
- 12.2.1 Famar SA
List of Figures
- Figure 1: Global Pharmaceutical CMO Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: North America Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 3: North America Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 4: North America Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 5: North America Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 6: Europe Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 7: Europe Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 8: Europe Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 9: Europe Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 10: Asia Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 11: Asia Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 12: Asia Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 13: Asia Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 14: Australia and New Zealand Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 15: Australia and New Zealand Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 16: Australia and New Zealand Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 17: Australia and New Zealand Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 18: Latin America Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 19: Latin America Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 20: Latin America Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 21: Latin America Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 22: Middle East and Africa Pharmaceutical CMO Market Revenue (Million), by Service Type 2025 & 2033
- Figure 23: Middle East and Africa Pharmaceutical CMO Market Revenue Share (%), by Service Type 2025 & 2033
- Figure 24: Middle East and Africa Pharmaceutical CMO Market Revenue (Million), by Country 2025 & 2033
- Figure 25: Middle East and Africa Pharmaceutical CMO Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 2: Global Pharmaceutical CMO Market Revenue Million Forecast, by Region 2020 & 2033
- Table 3: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 4: Global Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 5: United States Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 6: Canada Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 7: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 8: Global Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 9: United Kingdom Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 10: Germany Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 11: France Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 12: Italy Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 13: Spain Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 14: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 15: Global Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 16: China Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 17: India Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 18: Japan Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 19: Australia Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 20: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 21: Global Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 22: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 23: Global Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 24: Brazil Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 25: Mexico Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 26: Argentina Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 27: Global Pharmaceutical CMO Market Revenue Million Forecast, by Service Type 2020 & 2033
- Table 28: Global Pharmaceutical CMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 29: United Arab Emirates Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 30: Saudi Arabia Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 31: South Africa Pharmaceutical CMO Market Revenue (Million) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmaceutical CMO Market?
The projected CAGR is approximately 6.53%.
2. Which companies are prominent players in the Pharmaceutical CMO Market?
Key companies in the market include Famar SA, Lonza Group, Tesa Labtec GmbH (Tesa SE), ARX LL, Patheon Inc (Thermo Fisher Scientific Inc ), Pfizer CentreSource (Pfizer Inc ), Tapemark, Aenova Holdings GmbH, Catalent Inc, Boehringer Ingelheim Group, Recipharm AB, Baxter Biopharma Solutions (Baxter International Inc ), Jubilant Biosys Ltd (Jubilant Pharmova Ltd).
3. What are the main segments of the Pharmaceutical CMO Market?
The market segments include Service Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 173.13 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Outsourcing Volume by Pharmaceutical Companies; Increasing Investment in Research and Development.
6. What are the notable trends driving market growth?
Active Pharmaceutical Ingredient (API) and Intermediates are Expected to Witness Robust Demand.
7. Are there any restraints impacting market growth?
Increasing Lead Time and Logistics Costs; Stringent Regulatory Requirements; Capacity Utilization Issues Affecting the Profitability of CMOs.
8. Can you provide examples of recent developments in the market?
January 2023: Catalent announced that it had signed a development and license agreement with Ethicann Pharmaceuticals Inc., a Canadian/American specialty pharmaceutical company specializing in creating high-value cannabinoid drug therapies using Zydisorally disintegrating tablet (ODT) technology to advance Ethicann's clinical drug pipeline. Per the agreement, Catalent would use its Zydis technology to develop pharmaceutical products containing cannabidiol (CBD) and tetrahydrocannabinol (THC) for Ethicann's use in clinical trials for various conditions.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Pharmaceutical CMO Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Pharmaceutical CMO Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Pharmaceutical CMO Market?
To stay informed about further developments, trends, and reports in the Pharmaceutical CMO Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
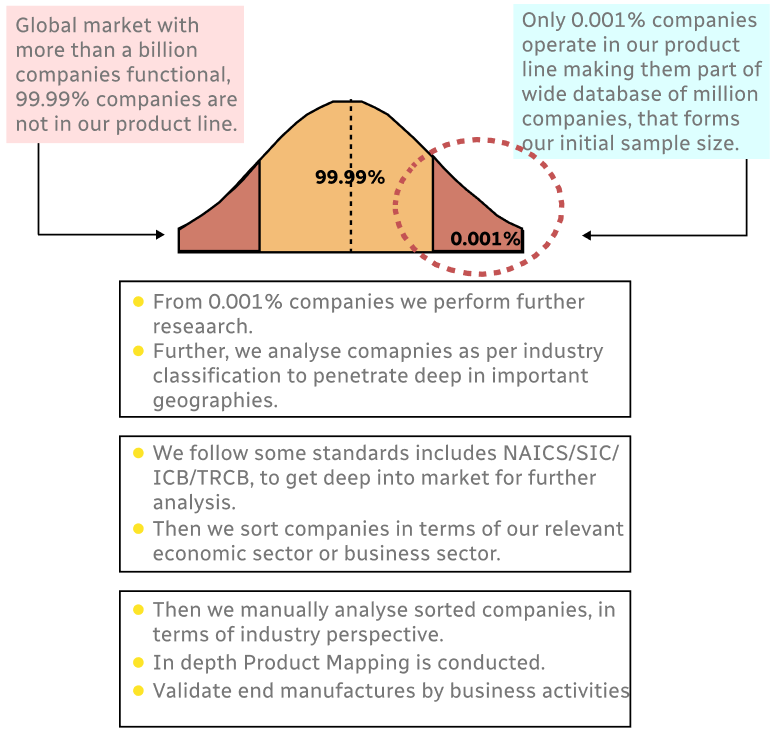
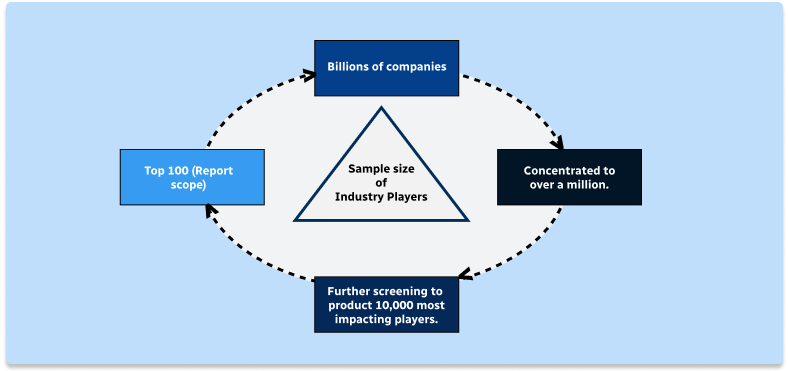
Step 1 - Identification of Relevant Samples Size from Population Database
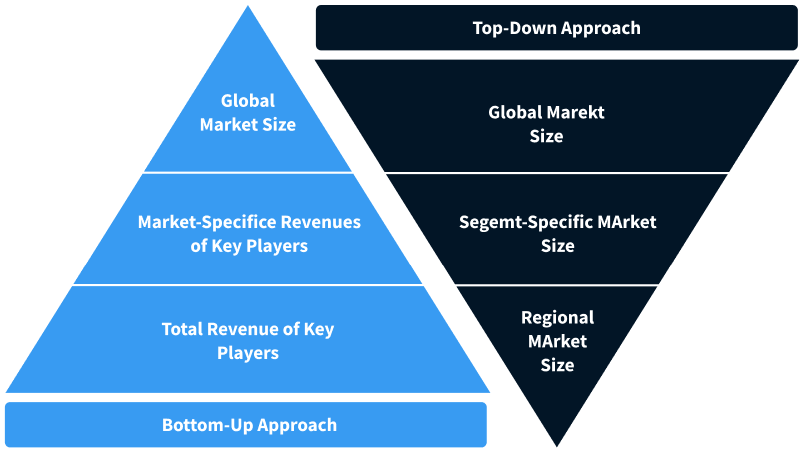
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


