Key Insights
The gene therapy market is poised for explosive growth, projected to reach approximately $7.18 billion in 2025 and expand at a remarkable Compound Annual Growth Rate (CAGR) of 28.00% through 2033. This surge is primarily driven by significant advancements in therapeutic technologies, particularly the increasing efficacy and safety profiles of Adeno-associated Virus (AAV) and Lentiviral vectors, which are becoming the preferred delivery mechanisms for a wide range of genetic disorders. The escalating prevalence of chronic and rare diseases, coupled with a growing understanding of genetic mechanisms, is fueling demand for novel treatment modalities. Furthermore, substantial investments in research and development by leading pharmaceutical and biotechnology companies, including F Hoffmann-La Roche Ltd (Spark Therapeutics), Astellas Pharma, and Novartis AG, are accelerating the pipeline of gene therapies for various indications. The market's expansion is further bolstered by favorable regulatory environments in key regions and increasing patient and physician awareness of the transformative potential of gene-based treatments.
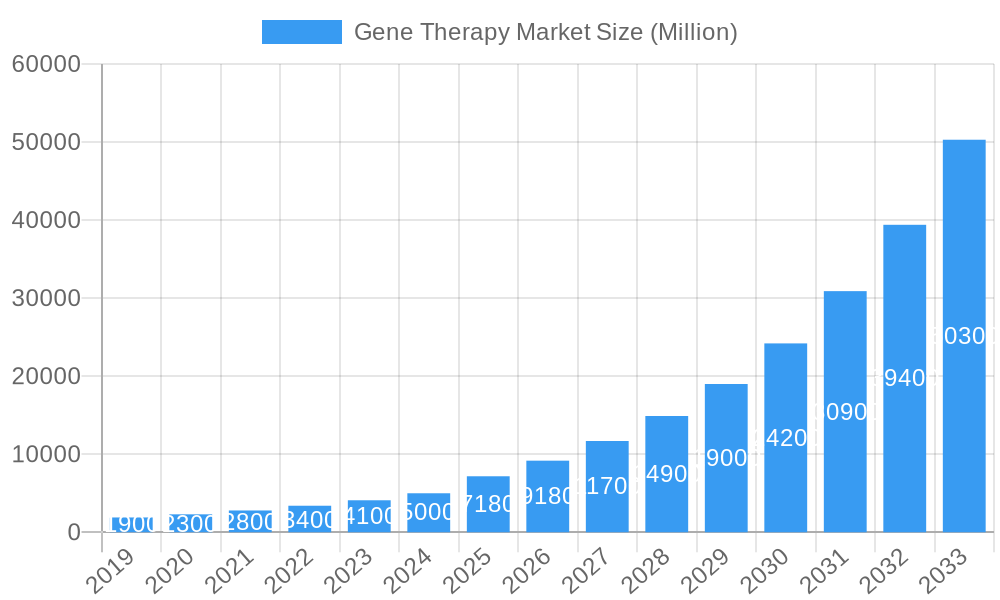
Gene Therapy Market Market Size (In Billion)
The market is segmented by both indication and technology, highlighting the diverse applications and innovative approaches within gene therapy. Indications such as Cancer, Metabolic Disorders, Eye Disorders, and Spinal Muscular Atrophy (SMA) represent major growth areas, driven by the unmet medical needs and the potential for curative or long-lasting treatments. For instance, gene therapies for certain cancers are showing promising results in clinical trials, while the successful commercialization of therapies for SMA has paved the way for broader adoption. On the technology front, AAV vectors are dominating due to their safety and efficiency, but research into Adenovirus and Lentiviral vectors continues to evolve, offering distinct advantages for specific therapeutic targets. Regionally, North America and Europe are currently leading the market, owing to robust healthcare infrastructure, high R&D spending, and early adoption of advanced therapies. However, the Asia Pacific region is expected to witness substantial growth, driven by a burgeoning biopharmaceutical sector, increasing healthcare expenditure, and a large patient population. The gene therapy market's trajectory is characterized by continuous innovation, strategic collaborations, and a commitment to addressing previously untreatable diseases, solidifying its position as a transformative force in modern medicine.
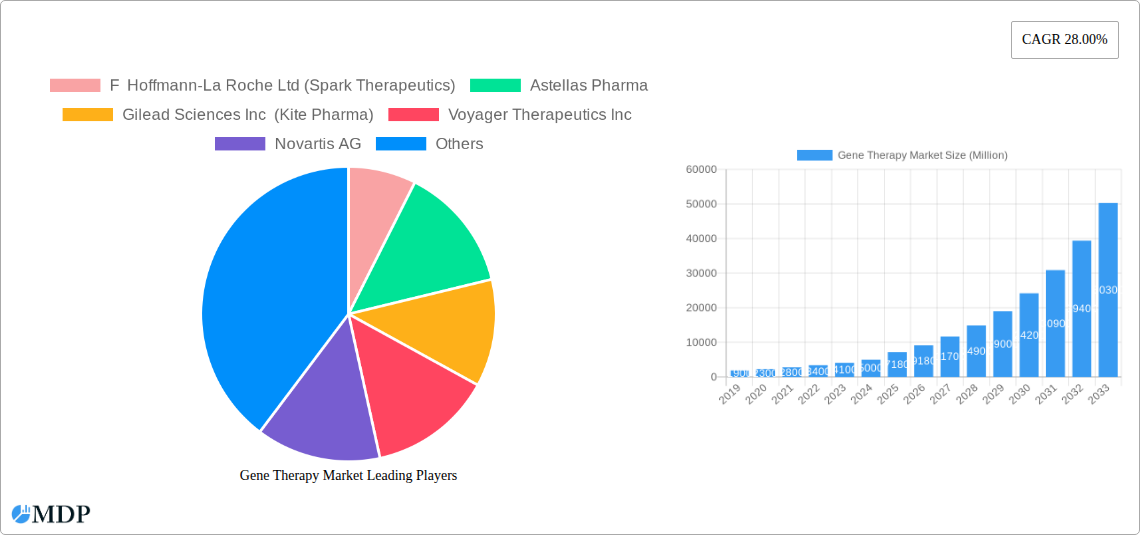
Gene Therapy Market Company Market Share
Explore the transformative Gene Therapy Market, a rapidly expanding frontier in healthcare poised to revolutionize disease treatment. This comprehensive report provides an in-depth analysis of the global gene therapy market, covering market dynamics, industry trends, leading segments, product developments, growth drivers, challenges, emerging opportunities, key players, and strategic outlook for the period 2019–2033, with a base and estimated year of 2025 and a forecast period of 2025–2033.
Gain actionable insights into market concentration, innovation drivers, and regulatory landscapes. Understand the trajectory of this multi-billion dollar industry, driven by cutting-edge technologies and a growing pipeline of groundbreaking therapies.
Gene Therapy Market Market Dynamics & Concentration
The gene therapy market is characterized by a dynamic interplay of innovation, strategic investments, and evolving regulatory frameworks. Market concentration is moderately high, with a few key players holding significant sway due to substantial R&D investments and established manufacturing capabilities. The primary innovation drivers stem from advancements in gene editing technologies like CRISPR-Cas9, improved vector delivery systems, and a deeper understanding of genetic diseases. Regulatory bodies, such as the US FDA and EMA, are actively adapting their guidelines to accommodate the unique nature of gene therapies, creating both pathways for approval and rigorous oversight. Product substitutes are limited in their ability to offer permanent cures for genetic disorders, but traditional treatments still represent a form of competition, especially for less severe conditions. End-user trends are increasingly focused on curative potential, personalized medicine approaches, and therapies addressing rare and previously untreatable diseases. Mergers and acquisitions (M&A) are a significant feature of this market, driven by the desire for synergistic capabilities, pipeline expansion, and access to novel technologies. For instance, strategic acquisitions by larger pharmaceutical companies aim to integrate promising gene therapy startups and their intellectual property. The M&A deal count has seen a steady rise, reflecting the high stakes and potential rewards in this sector.
- Market Concentration: Moderate to High, driven by R&D intensity and specialized manufacturing.
- Innovation Drivers: CRISPR-Cas9, advanced viral vectors, precision medicine, understanding of genetic pathways.
- Regulatory Frameworks: Evolving, with dedicated pathways for advanced therapies, rigorous safety and efficacy evaluations.
- Product Substitutes: Primarily traditional treatments and supportive care; limited curative substitutes.
- End-User Trends: Demand for curative therapies, personalized medicine, treatment of rare diseases, improved quality of life.
- M&A Activities: Active, with acquisitions focused on technology, pipeline, and market access.
Gene Therapy Market Industry Trends & Analysis
The global gene therapy market is experiencing robust growth, projected to achieve a Compound Annual Growth Rate (CAGR) of XX% from 2025 to 2033, reaching an estimated market size of over $30,000 million by 2033. This expansion is fueled by a confluence of factors, including a growing understanding of the genetic basis of diseases, significant breakthroughs in gene editing and delivery technologies, and increasing investment from both public and private sectors. The development of sophisticated delivery vectors, such as adeno-associated viruses (AAVs) and lentiviral vectors, has been pivotal in enhancing the safety and efficacy of gene therapies, enabling targeted delivery to specific cell types. Technological disruptions, particularly the advent of gene editing tools like CRISPR-Cas9, have opened up unprecedented possibilities for correcting disease-causing genetic mutations, moving beyond simply replacing or supplementing faulty genes. Consumer preferences are shifting towards therapies that offer a potential one-time cure rather than lifelong management of chronic conditions. This is particularly evident in the demand for gene therapies treating rare genetic disorders that have limited or no effective treatment options. The competitive dynamics within the market are intensifying, with both established pharmaceutical giants and agile biotechnology startups vying for market share through the development of novel therapies and strategic partnerships. Key therapeutic areas like oncology, rare genetic diseases, and neurological disorders are witnessing significant investment and a surge in clinical trials. The market penetration of gene therapies is steadily increasing as more products gain regulatory approval and become accessible to patients, albeit often at high price points that reflect the complex R&D and manufacturing processes. The increasing prevalence of genetic diseases and the aging global population also contribute to the sustained demand for innovative treatment modalities like gene therapy. Furthermore, advancements in manufacturing technologies are helping to scale up production, which is crucial for meeting the growing demand.
Leading Markets & Segments in Gene Therapy Market
The Gene Therapy Market is segmented across various indications and technologies, each with distinct growth drivers and market dominance. North America, particularly the United States, currently leads the market, driven by robust R&D infrastructure, favorable regulatory pathways for innovative therapies, and substantial private and public funding. The United States' dominance is further amplified by its large patient population and the presence of leading gene therapy developers.
Indication-wise Dominance:
- Cancer: This segment is a significant driver of market growth, with a high number of ongoing clinical trials and approved therapies, particularly CAR T-cell therapies. The unmet need for effective cancer treatments and the potential for long-term remission contribute to its leading position.
- Spinal Muscular Atrophy (SMA): Gene therapies for SMA have demonstrated remarkable efficacy, leading to substantial market penetration and setting a precedent for other rare neurological disorders.
- Metabolic Disorders: With increasing understanding of the genetic roots of metabolic diseases, this segment is poised for significant growth, driven by the potential for curative interventions.
- Eye Disorders: Early successes in gene therapies for inherited retinal diseases have established this segment as a key area of development and market value.
- Other Indications: This broad category encompasses a range of rare genetic disorders and infectious diseases, with strong potential for future expansion as research progresses.
Technology-wise Dominance:
- Adeno-associated Virus (AAV) Vector: AAV vectors are currently the most prevalent technology due to their favorable safety profile, broad tropism, and ability to transduce both dividing and non-dividing cells. Their widespread use in approved therapies and ongoing clinical trials solidifies their leadership.
- Lentiviral Vector: Lentiviral vectors are crucial for applications requiring stable integration into the host genome, particularly in ex vivo gene therapies for hematological disorders.
- Adeno Virus Vector: While facing some immunogenicity challenges, Adenovirus vectors remain relevant for certain applications due to their high payload capacity.
- Retroviral Vector: Historically significant, Retroviral vectors are still utilized in specific research and therapeutic contexts.
- Herpes Virus Vector and Other Technologies: These represent emerging or niche technologies with specialized applications and ongoing development.
The economic policies in leading countries, such as tax incentives for R&D and government funding for rare disease research, play a crucial role in market expansion. Infrastructure development, including specialized manufacturing facilities and clinical trial networks, further supports the growth of dominant segments.
Gene Therapy Market Product Developments
The gene therapy market is witnessing an influx of innovative product developments aimed at addressing a wide spectrum of genetic and acquired diseases. Recent advancements include next-generation viral vector technologies offering improved safety and targeting capabilities, alongside the exploration of non-viral delivery systems to overcome some of the limitations associated with viral vectors. Gene editing technologies are continuously being refined to enhance precision and reduce off-target effects. Products are increasingly designed for in vivo administration, simplifying treatment protocols and expanding accessibility. The competitive advantage lies in achieving durable clinical outcomes, demonstrating a favorable safety profile, and offering a potential one-time curative treatment for conditions that were previously managed with lifelong therapies. The market fit for these products is strong for rare genetic disorders, certain cancers, and conditions with significant unmet medical needs, driving substantial research and development efforts.
Key Drivers of Gene Therapy Market Growth
The gene therapy market's growth is propelled by several powerful drivers. Technological Advancements in gene editing (e.g., CRISPR), vector engineering, and synthetic biology are continuously expanding the possibilities for treating genetic diseases. Increasing R&D Investments from pharmaceutical companies, biotech firms, and venture capital underscore the perceived potential of gene therapies. Furthermore, Favorable Regulatory Pathways established by agencies like the FDA and EMA are accelerating the approval of innovative gene therapies. Growing Prevalence of Rare and Genetic Diseases creates a significant unmet medical need, driving demand for curative solutions. Finally, Technological Advancements in Manufacturing are making it more feasible and cost-effective to produce these complex therapies at scale.
Challenges in the Gene Therapy Market Market
Despite its immense promise, the gene therapy market faces several significant challenges. The High Cost of Treatment remains a major barrier, limiting patient access and posing challenges for healthcare reimbursement systems, with some therapies exceeding $2 million per treatment. Regulatory Hurdles related to long-term safety and efficacy data, as well as the complexity of manufacturing and quality control, can delay approvals. Manufacturing and Scalability Issues present logistical complexities, requiring specialized facilities and expertise to produce therapies consistently and at sufficient volumes. Immunogenicity Concerns related to viral vectors can lead to adverse patient reactions and limit re-dosing potential. Finally, Ethical Considerations surrounding germline gene editing and equitable access also need careful navigation.
Emerging Opportunities in Gene Therapy Market
Emerging opportunities in the gene therapy market are abundant and poised to drive long-term growth. Technological Breakthroughs in areas like gene editing precision, synthetic biology for novel vectors, and the development of efficient non-viral delivery systems are opening up new therapeutic avenues. Strategic Partnerships and Collaborations between academic institutions, biotech startups, and large pharmaceutical companies are accelerating drug discovery and development. Market Expansion into New Indications beyond rare genetic diseases, such as common chronic conditions and infectious diseases, presents significant growth potential. Furthermore, the development of "Off-the-Shelf" Allogeneic Therapies could address scalability and cost challenges associated with autologous approaches. Advances in Personalized Medicine and companion diagnostics are also creating opportunities for more targeted and effective gene therapies.
Leading Players in the Gene Therapy Market Sector
- F Hoffmann-La Roche Ltd (Spark Therapeutics)
- Astellas Pharma
- Gilead Sciences Inc (Kite Pharma)
- Voyager Therapeutics Inc
- Novartis AG
- Amgen Inc
- UniQure NV
- Generation Bio
- Abeona Therapeutics Inc
- Bluebird Bio Inc
- Poseida Therapeutics
- Biogen Inc
Key Milestones in Gene Therapy Market Industry
- October 2022: Sarepta Therapeutics applied to the US FDA for accelerated approval of delandistrogene moxeparvovec for Duchenne Muscular Dystrophy (DMD).
- August 2022: The US FDA approved Zynteglo (betibeglogene autotemcel), the first cell-based gene therapy for beta-thalassemia requiring regular red blood cell transfusions in adult and pediatric patients.
Strategic Outlook for Gene Therapy Market Market
The strategic outlook for the gene therapy market is exceptionally strong, driven by an anticipated surge in therapeutic approvals and market penetration. Key growth accelerators include the continued advancement of gene editing technologies, leading to more precise and effective treatments. The expansion of manufacturing capabilities and the development of more cost-effective production methods will be crucial for improving accessibility. Strategic partnerships and collaborations will continue to play a vital role in de-risking R&D and accelerating pipeline development. Furthermore, a focus on expanding the therapeutic scope beyond rare genetic diseases into more prevalent conditions will unlock significant future market potential. The ongoing refinement of regulatory pathways and robust investment from public and private sectors will further fuel this transformative industry.
Gene Therapy Market Segmentation
-
1. Indication
- 1.1. Cancer
- 1.2. Metabolic Disorders
- 1.3. Eye Disorders
- 1.4. Spinal Muscular Atrophy
- 1.5. Other Indications
-
2. Technology
- 2.1. Adeno Virus Vector
- 2.2. Adeno-associated Virus Vector
- 2.3. Lentiviral Vector
- 2.4. Retroviral Vector
- 2.5. Herpes Virus Vector
- 2.6. Other Technologies
Gene Therapy Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
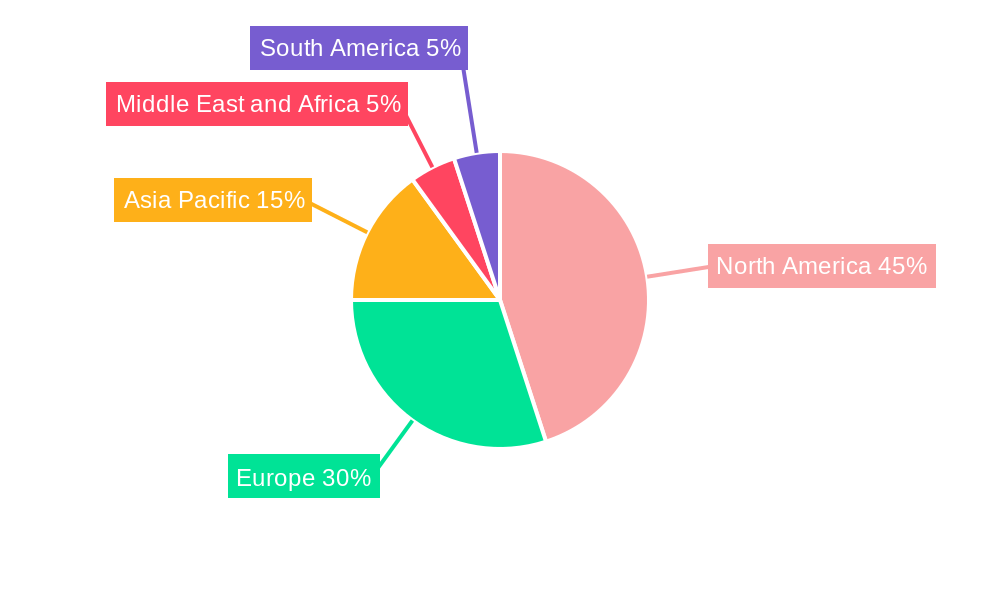
Gene Therapy Market Regional Market Share
Geographic Coverage of Gene Therapy Market
Gene Therapy Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 28.00% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Investments in Research and Development; Technological Advancements; Growing Prevalence of Target Diseases like Cancer
- 3.3. Market Restrains
- 3.3.1. Lack of Standard Regulations; High Price of Products
- 3.4. Market Trends
- 3.4.1. Cancer is Expected to Hold Significant Market Share in the Indication Segment over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Gene Therapy Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Indication
- 5.1.1. Cancer
- 5.1.2. Metabolic Disorders
- 5.1.3. Eye Disorders
- 5.1.4. Spinal Muscular Atrophy
- 5.1.5. Other Indications
- 5.2. Market Analysis, Insights and Forecast - by Technology
- 5.2.1. Adeno Virus Vector
- 5.2.2. Adeno-associated Virus Vector
- 5.2.3. Lentiviral Vector
- 5.2.4. Retroviral Vector
- 5.2.5. Herpes Virus Vector
- 5.2.6. Other Technologies
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Middle East and Africa
- 5.3.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Indication
- 6. North America Gene Therapy Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Indication
- 6.1.1. Cancer
- 6.1.2. Metabolic Disorders
- 6.1.3. Eye Disorders
- 6.1.4. Spinal Muscular Atrophy
- 6.1.5. Other Indications
- 6.2. Market Analysis, Insights and Forecast - by Technology
- 6.2.1. Adeno Virus Vector
- 6.2.2. Adeno-associated Virus Vector
- 6.2.3. Lentiviral Vector
- 6.2.4. Retroviral Vector
- 6.2.5. Herpes Virus Vector
- 6.2.6. Other Technologies
- 6.1. Market Analysis, Insights and Forecast - by Indication
- 7. Europe Gene Therapy Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Indication
- 7.1.1. Cancer
- 7.1.2. Metabolic Disorders
- 7.1.3. Eye Disorders
- 7.1.4. Spinal Muscular Atrophy
- 7.1.5. Other Indications
- 7.2. Market Analysis, Insights and Forecast - by Technology
- 7.2.1. Adeno Virus Vector
- 7.2.2. Adeno-associated Virus Vector
- 7.2.3. Lentiviral Vector
- 7.2.4. Retroviral Vector
- 7.2.5. Herpes Virus Vector
- 7.2.6. Other Technologies
- 7.1. Market Analysis, Insights and Forecast - by Indication
- 8. Asia Pacific Gene Therapy Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Indication
- 8.1.1. Cancer
- 8.1.2. Metabolic Disorders
- 8.1.3. Eye Disorders
- 8.1.4. Spinal Muscular Atrophy
- 8.1.5. Other Indications
- 8.2. Market Analysis, Insights and Forecast - by Technology
- 8.2.1. Adeno Virus Vector
- 8.2.2. Adeno-associated Virus Vector
- 8.2.3. Lentiviral Vector
- 8.2.4. Retroviral Vector
- 8.2.5. Herpes Virus Vector
- 8.2.6. Other Technologies
- 8.1. Market Analysis, Insights and Forecast - by Indication
- 9. Middle East and Africa Gene Therapy Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Indication
- 9.1.1. Cancer
- 9.1.2. Metabolic Disorders
- 9.1.3. Eye Disorders
- 9.1.4. Spinal Muscular Atrophy
- 9.1.5. Other Indications
- 9.2. Market Analysis, Insights and Forecast - by Technology
- 9.2.1. Adeno Virus Vector
- 9.2.2. Adeno-associated Virus Vector
- 9.2.3. Lentiviral Vector
- 9.2.4. Retroviral Vector
- 9.2.5. Herpes Virus Vector
- 9.2.6. Other Technologies
- 9.1. Market Analysis, Insights and Forecast - by Indication
- 10. South America Gene Therapy Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Indication
- 10.1.1. Cancer
- 10.1.2. Metabolic Disorders
- 10.1.3. Eye Disorders
- 10.1.4. Spinal Muscular Atrophy
- 10.1.5. Other Indications
- 10.2. Market Analysis, Insights and Forecast - by Technology
- 10.2.1. Adeno Virus Vector
- 10.2.2. Adeno-associated Virus Vector
- 10.2.3. Lentiviral Vector
- 10.2.4. Retroviral Vector
- 10.2.5. Herpes Virus Vector
- 10.2.6. Other Technologies
- 10.1. Market Analysis, Insights and Forecast - by Indication
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 F Hoffmann-La Roche Ltd (Spark Therapeutics)
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Astellas Pharma
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Gilead Sciences Inc (Kite Pharma)
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Voyager Therapeutics Inc
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Novartis AG
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Amgen Inc
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 UniQure NV
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Generation Bio
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Abeona Therapeutics Inc
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Bluebird Bio Inc
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Poseida Therapeutics
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Biogen Inc
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.1 F Hoffmann-La Roche Ltd (Spark Therapeutics)
List of Figures
- Figure 1: Global Gene Therapy Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: Global Gene Therapy Market Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Gene Therapy Market Revenue (Million), by Indication 2025 & 2033
- Figure 4: North America Gene Therapy Market Volume (K Unit), by Indication 2025 & 2033
- Figure 5: North America Gene Therapy Market Revenue Share (%), by Indication 2025 & 2033
- Figure 6: North America Gene Therapy Market Volume Share (%), by Indication 2025 & 2033
- Figure 7: North America Gene Therapy Market Revenue (Million), by Technology 2025 & 2033
- Figure 8: North America Gene Therapy Market Volume (K Unit), by Technology 2025 & 2033
- Figure 9: North America Gene Therapy Market Revenue Share (%), by Technology 2025 & 2033
- Figure 10: North America Gene Therapy Market Volume Share (%), by Technology 2025 & 2033
- Figure 11: North America Gene Therapy Market Revenue (Million), by Country 2025 & 2033
- Figure 12: North America Gene Therapy Market Volume (K Unit), by Country 2025 & 2033
- Figure 13: North America Gene Therapy Market Revenue Share (%), by Country 2025 & 2033
- Figure 14: North America Gene Therapy Market Volume Share (%), by Country 2025 & 2033
- Figure 15: Europe Gene Therapy Market Revenue (Million), by Indication 2025 & 2033
- Figure 16: Europe Gene Therapy Market Volume (K Unit), by Indication 2025 & 2033
- Figure 17: Europe Gene Therapy Market Revenue Share (%), by Indication 2025 & 2033
- Figure 18: Europe Gene Therapy Market Volume Share (%), by Indication 2025 & 2033
- Figure 19: Europe Gene Therapy Market Revenue (Million), by Technology 2025 & 2033
- Figure 20: Europe Gene Therapy Market Volume (K Unit), by Technology 2025 & 2033
- Figure 21: Europe Gene Therapy Market Revenue Share (%), by Technology 2025 & 2033
- Figure 22: Europe Gene Therapy Market Volume Share (%), by Technology 2025 & 2033
- Figure 23: Europe Gene Therapy Market Revenue (Million), by Country 2025 & 2033
- Figure 24: Europe Gene Therapy Market Volume (K Unit), by Country 2025 & 2033
- Figure 25: Europe Gene Therapy Market Revenue Share (%), by Country 2025 & 2033
- Figure 26: Europe Gene Therapy Market Volume Share (%), by Country 2025 & 2033
- Figure 27: Asia Pacific Gene Therapy Market Revenue (Million), by Indication 2025 & 2033
- Figure 28: Asia Pacific Gene Therapy Market Volume (K Unit), by Indication 2025 & 2033
- Figure 29: Asia Pacific Gene Therapy Market Revenue Share (%), by Indication 2025 & 2033
- Figure 30: Asia Pacific Gene Therapy Market Volume Share (%), by Indication 2025 & 2033
- Figure 31: Asia Pacific Gene Therapy Market Revenue (Million), by Technology 2025 & 2033
- Figure 32: Asia Pacific Gene Therapy Market Volume (K Unit), by Technology 2025 & 2033
- Figure 33: Asia Pacific Gene Therapy Market Revenue Share (%), by Technology 2025 & 2033
- Figure 34: Asia Pacific Gene Therapy Market Volume Share (%), by Technology 2025 & 2033
- Figure 35: Asia Pacific Gene Therapy Market Revenue (Million), by Country 2025 & 2033
- Figure 36: Asia Pacific Gene Therapy Market Volume (K Unit), by Country 2025 & 2033
- Figure 37: Asia Pacific Gene Therapy Market Revenue Share (%), by Country 2025 & 2033
- Figure 38: Asia Pacific Gene Therapy Market Volume Share (%), by Country 2025 & 2033
- Figure 39: Middle East and Africa Gene Therapy Market Revenue (Million), by Indication 2025 & 2033
- Figure 40: Middle East and Africa Gene Therapy Market Volume (K Unit), by Indication 2025 & 2033
- Figure 41: Middle East and Africa Gene Therapy Market Revenue Share (%), by Indication 2025 & 2033
- Figure 42: Middle East and Africa Gene Therapy Market Volume Share (%), by Indication 2025 & 2033
- Figure 43: Middle East and Africa Gene Therapy Market Revenue (Million), by Technology 2025 & 2033
- Figure 44: Middle East and Africa Gene Therapy Market Volume (K Unit), by Technology 2025 & 2033
- Figure 45: Middle East and Africa Gene Therapy Market Revenue Share (%), by Technology 2025 & 2033
- Figure 46: Middle East and Africa Gene Therapy Market Volume Share (%), by Technology 2025 & 2033
- Figure 47: Middle East and Africa Gene Therapy Market Revenue (Million), by Country 2025 & 2033
- Figure 48: Middle East and Africa Gene Therapy Market Volume (K Unit), by Country 2025 & 2033
- Figure 49: Middle East and Africa Gene Therapy Market Revenue Share (%), by Country 2025 & 2033
- Figure 50: Middle East and Africa Gene Therapy Market Volume Share (%), by Country 2025 & 2033
- Figure 51: South America Gene Therapy Market Revenue (Million), by Indication 2025 & 2033
- Figure 52: South America Gene Therapy Market Volume (K Unit), by Indication 2025 & 2033
- Figure 53: South America Gene Therapy Market Revenue Share (%), by Indication 2025 & 2033
- Figure 54: South America Gene Therapy Market Volume Share (%), by Indication 2025 & 2033
- Figure 55: South America Gene Therapy Market Revenue (Million), by Technology 2025 & 2033
- Figure 56: South America Gene Therapy Market Volume (K Unit), by Technology 2025 & 2033
- Figure 57: South America Gene Therapy Market Revenue Share (%), by Technology 2025 & 2033
- Figure 58: South America Gene Therapy Market Volume Share (%), by Technology 2025 & 2033
- Figure 59: South America Gene Therapy Market Revenue (Million), by Country 2025 & 2033
- Figure 60: South America Gene Therapy Market Volume (K Unit), by Country 2025 & 2033
- Figure 61: South America Gene Therapy Market Revenue Share (%), by Country 2025 & 2033
- Figure 62: South America Gene Therapy Market Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Gene Therapy Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 2: Global Gene Therapy Market Volume K Unit Forecast, by Indication 2020 & 2033
- Table 3: Global Gene Therapy Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 4: Global Gene Therapy Market Volume K Unit Forecast, by Technology 2020 & 2033
- Table 5: Global Gene Therapy Market Revenue Million Forecast, by Region 2020 & 2033
- Table 6: Global Gene Therapy Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 7: Global Gene Therapy Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 8: Global Gene Therapy Market Volume K Unit Forecast, by Indication 2020 & 2033
- Table 9: Global Gene Therapy Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 10: Global Gene Therapy Market Volume K Unit Forecast, by Technology 2020 & 2033
- Table 11: Global Gene Therapy Market Revenue Million Forecast, by Country 2020 & 2033
- Table 12: Global Gene Therapy Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 13: United States Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 14: United States Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 15: Canada Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 16: Canada Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 17: Mexico Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 18: Mexico Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Global Gene Therapy Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 20: Global Gene Therapy Market Volume K Unit Forecast, by Indication 2020 & 2033
- Table 21: Global Gene Therapy Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 22: Global Gene Therapy Market Volume K Unit Forecast, by Technology 2020 & 2033
- Table 23: Global Gene Therapy Market Revenue Million Forecast, by Country 2020 & 2033
- Table 24: Global Gene Therapy Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 25: Germany Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 26: Germany Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 27: United Kingdom Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 28: United Kingdom Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 29: France Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 30: France Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 31: Italy Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 32: Italy Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: Spain Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 34: Spain Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: Rest of Europe Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 36: Rest of Europe Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Global Gene Therapy Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 38: Global Gene Therapy Market Volume K Unit Forecast, by Indication 2020 & 2033
- Table 39: Global Gene Therapy Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 40: Global Gene Therapy Market Volume K Unit Forecast, by Technology 2020 & 2033
- Table 41: Global Gene Therapy Market Revenue Million Forecast, by Country 2020 & 2033
- Table 42: Global Gene Therapy Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 43: China Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 44: China Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 45: Japan Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 46: Japan Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 47: India Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 48: India Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 49: Australia Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 50: Australia Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 51: South Korea Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 52: South Korea Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Rest of Asia Pacific Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 54: Rest of Asia Pacific Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: Global Gene Therapy Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 56: Global Gene Therapy Market Volume K Unit Forecast, by Indication 2020 & 2033
- Table 57: Global Gene Therapy Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 58: Global Gene Therapy Market Volume K Unit Forecast, by Technology 2020 & 2033
- Table 59: Global Gene Therapy Market Revenue Million Forecast, by Country 2020 & 2033
- Table 60: Global Gene Therapy Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 61: GCC Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 62: GCC Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: South Africa Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 64: South Africa Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 65: Rest of Middle East and Africa Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 66: Rest of Middle East and Africa Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 67: Global Gene Therapy Market Revenue Million Forecast, by Indication 2020 & 2033
- Table 68: Global Gene Therapy Market Volume K Unit Forecast, by Indication 2020 & 2033
- Table 69: Global Gene Therapy Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 70: Global Gene Therapy Market Volume K Unit Forecast, by Technology 2020 & 2033
- Table 71: Global Gene Therapy Market Revenue Million Forecast, by Country 2020 & 2033
- Table 72: Global Gene Therapy Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 73: Brazil Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 74: Brazil Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Argentina Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 76: Argentina Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Rest of South America Gene Therapy Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 78: Rest of South America Gene Therapy Market Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Gene Therapy Market?
The projected CAGR is approximately 28.00%.
2. Which companies are prominent players in the Gene Therapy Market?
Key companies in the market include F Hoffmann-La Roche Ltd (Spark Therapeutics), Astellas Pharma, Gilead Sciences Inc (Kite Pharma), Voyager Therapeutics Inc , Novartis AG, Amgen Inc, UniQure NV, Generation Bio, Abeona Therapeutics Inc, Bluebird Bio Inc, Poseida Therapeutics, Biogen Inc.
3. What are the main segments of the Gene Therapy Market?
The market segments include Indication, Technology.
4. Can you provide details about the market size?
The market size is estimated to be USD 7.18 Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Investments in Research and Development; Technological Advancements; Growing Prevalence of Target Diseases like Cancer.
6. What are the notable trends driving market growth?
Cancer is Expected to Hold Significant Market Share in the Indication Segment over the Forecast Period.
7. Are there any restraints impacting market growth?
Lack of Standard Regulations; High Price of Products.
8. Can you provide examples of recent developments in the market?
In October 2022, Sarepta Therapeutics applied to the US FDA to grant accelerated approval to the gene therapy S (delandistrogene moxeparvovec) for the treatment of Duchenne Muscular Dystrophy (DMD).
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Gene Therapy Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Gene Therapy Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Gene Therapy Market?
To stay informed about further developments, trends, and reports in the Gene Therapy Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
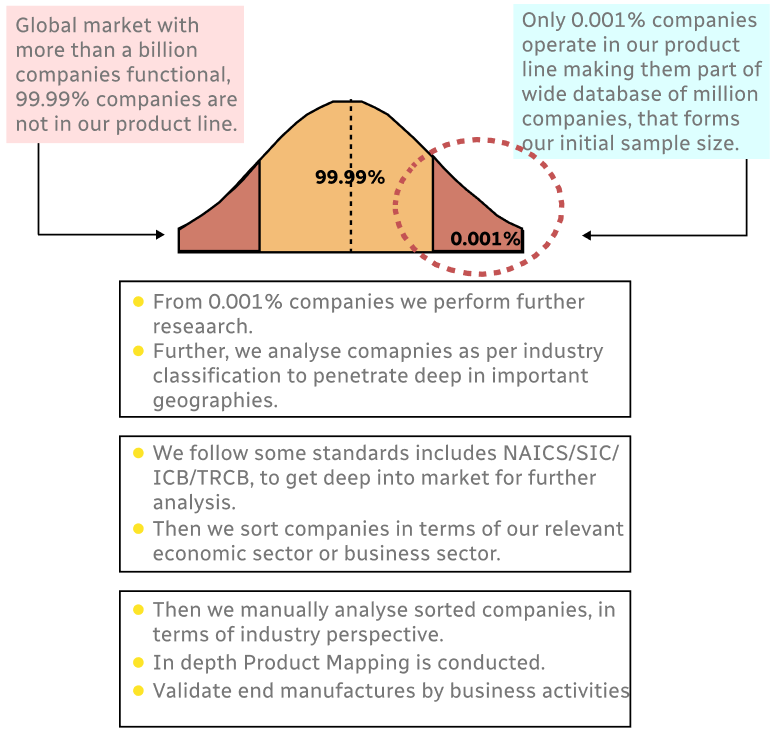
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


