Key Insights
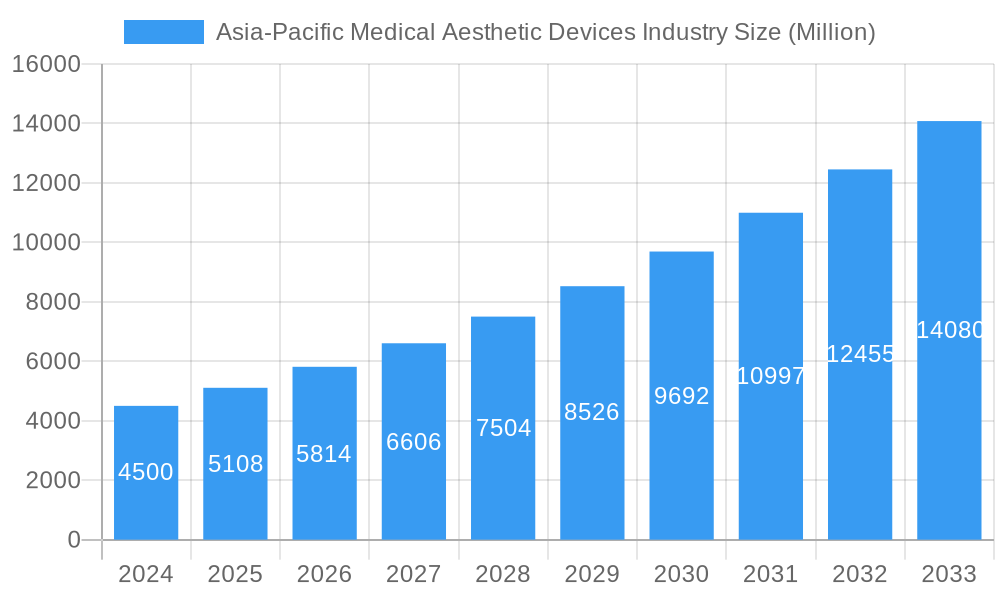
The Asia-Pacific Medical Aesthetic Devices market is poised for remarkable expansion, with a current estimated market size of USD 4.5 billion in 2024. The industry is projected to witness a robust CAGR of 13.8% during the forecast period of 2025-2033, indicating a dynamic and rapidly evolving landscape. This growth is primarily fueled by increasing consumer disposable income, a rising awareness and acceptance of aesthetic procedures, and advancements in medical technology. The region's burgeoning middle class, coupled with a strong desire for improved appearance and anti-aging solutions, is a significant catalyst for demand. Furthermore, a growing preference for minimally invasive and non-invasive procedures is driving the adoption of energy-based aesthetic devices, such as laser and radiofrequency technologies, which offer enhanced safety and quicker recovery times. The competitive landscape features prominent players like Sciton Inc., Candela Corporation, and Hologic Inc., constantly innovating to meet evolving consumer needs.
Asia-Pacific Medical Aesthetic Devices Industry Market Size (In Billion)
The market segmentation reveals a diverse range of applications and device types contributing to this growth. Skin resurfacing and tightening, along with body contouring and cellulite reduction, are identified as key application areas. Within device types, both energy-based and non-energy-based aesthetic devices are experiencing substantial traction. Non-energy-based devices, including popular treatments like Botulinum Toxin and dermal fillers, are gaining popularity due to their accessibility and immediate results. Conversely, energy-based devices are witnessing advancements in efficacy and patient comfort, appealing to a broader demographic. The increasing adoption of these devices in clinics and hospitals, alongside a nascent but growing trend in home-use devices, suggests a multi-faceted market expansion. Geographically, China, Japan, and South Korea are anticipated to remain dominant markets, driven by their advanced healthcare infrastructure and high consumer spending on aesthetic treatments, while emerging economies like India are expected to contribute significantly to future growth.
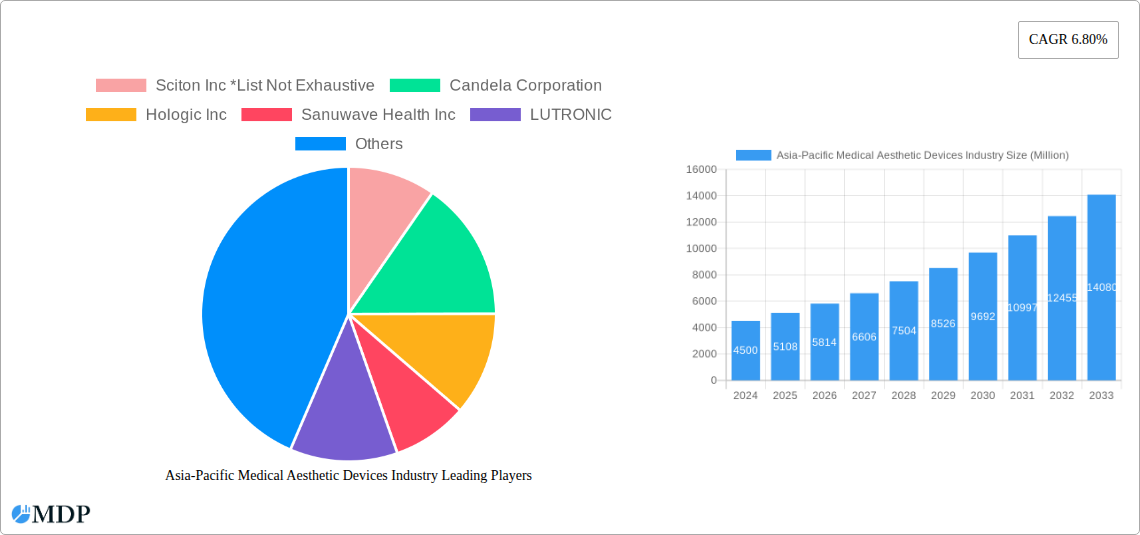
Asia-Pacific Medical Aesthetic Devices Industry Company Market Share
This in-depth report provides an authoritative analysis of the Asia-Pacific medical aesthetic devices market, a dynamic sector projected to reach over \$50 billion by 2025. Delving into the intricate landscape from 2019 to 2033, with a focus on the base year 2025 and a forecast period of 2025–2033, this study offers unparalleled insights into market dynamics, key trends, leading players, and emerging opportunities. We meticulously examine the growth of energy-based aesthetic devices (including laser-based aesthetic devices, radiofrequency (RF)-based aesthetic devices, light-based aesthetic devices, and ultrasound aesthetic devices) and non-energy-based aesthetic devices (such as Botulinum Toxin, Dermal Fillers and Aesthetic Threads, Chemical Peels, Microdermabrasion, and Implants including facial implants, breast implants, and other implants), alongside their applications in skin resurfacing and tightening, body contouring and cellulite reduction, hair removal, tattoo removal, and breast augmentation. Discover critical market intelligence for stakeholders in hospitals, clinics, and the burgeoning home settings across major geographies including China, Japan, India, Australia, South Korea, and the Rest of Asia-Pacific.
Asia-Pacific Medical Aesthetic Devices Industry Market Dynamics & Concentration
The Asia-Pacific medical aesthetic devices market is characterized by a moderate to high concentration, with a few key players holding significant market share, particularly in the energy-based aesthetic devices segment. Innovation is a primary driver, fueled by advancements in laser technology, radiofrequency applications, and the development of minimally invasive non-energy-based aesthetic devices like advanced dermal fillers. Regulatory frameworks are evolving, with increasing scrutiny on device safety and efficacy, impacting product development timelines and market entry strategies. The availability of effective product substitutes, such as advanced skincare formulations, presents a competitive challenge, though the demand for advanced aesthetic procedures remains robust. End-user trends indicate a growing demand for natural-looking results and personalized treatments, driving innovation in device customization and patient-specific applications. Mergers and acquisitions (M&A) activity is expected to remain steady, as larger companies seek to expand their product portfolios and geographical reach. Anticipated M&A deal counts are estimated to be around 10-15 annually during the forecast period, with major acquisitions focusing on companies with novel technologies in skin tightening and body contouring.
Asia-Pacific Medical Aesthetic Devices Industry Industry Trends & Analysis
The Asia-Pacific medical aesthetic devices market is poised for substantial growth, driven by a confluence of factors including rising disposable incomes, increasing consumer awareness of aesthetic treatments, and the growing influence of social media on beauty standards. The CAGR for the overall market is projected to be approximately 8.5% during the forecast period, indicating robust expansion. Technological disruptions are a constant feature, with innovations in energy-based devices offering enhanced precision, reduced downtime, and improved patient outcomes. For instance, advancements in ultrasound aesthetic devices for body contouring are gaining traction. Consumer preferences are shifting towards non-surgical and minimally invasive procedures, boosting the demand for dermal fillers, Botulinum Toxin, and advanced laser-based aesthetic devices for skin resurfacing. The competitive dynamics are intensifying, with both established global players and innovative regional manufacturers vying for market share. Market penetration for aesthetic devices is steadily increasing, particularly in urban centers across China, India, and South Korea, where a growing middle class actively seeks aesthetic enhancements. The increasing adoption of sophisticated devices in clinics and specialized hospitals further fuels this trend.
Leading Markets & Segments in Asia-Pacific Medical Aesthetic Devices Industry
China stands out as the dominant market within the Asia-Pacific region for medical aesthetic devices, driven by its large population, rapidly expanding middle class, and a significant appetite for cosmetic enhancements. The energy-based aesthetic device segment, particularly laser-based aesthetic devices for skin resurfacing and tightening and light-based aesthetic devices for hair removal, are leading the charge.
Dominant Segment: Energy-Based Aesthetic Devices:
- Laser-based Aesthetic Devices: These are highly sought after for applications such as tattoo removal, skin resurfacing, and hair removal, owing to their efficacy and versatility.
- Radiofrequency (RF)-based Aesthetic Devices: Their popularity is surging for skin tightening and body contouring, offering non-invasive solutions with minimal downtime.
- Light-based Aesthetic Devices: Widely used for hair removal and general skin rejuvenation.
- Ultrasound Aesthetic Devices: Emerging strong for body contouring and cellulite reduction, offering deep tissue treatment.
Key Application Dominance:
- Skin Resurfacing and Tightening: This application consistently tops the charts due to increasing demand for anti-aging solutions and the prevalence of skin concerns like acne scars and pigmentation.
- Body Contouring and Cellulite Reduction: Growing awareness and the desire for sculpted physiques are propelling this segment forward, with advancements in both energy-based and non-energy-based treatments.
End-User Landscape:
- Clinics: Represent the largest end-user segment, as most aesthetic procedures are performed in specialized clinics, offering accessibility and specialized care.
- Hospitals: While a smaller segment, hospitals are increasingly investing in advanced aesthetic devices for reconstructive and cosmetic surgeries.
- Home Settings: The rise of at-home devices, particularly for skincare and minor treatments, is a growing trend, though still nascent compared to professional settings.
The Rest of Asia-Pacific also presents significant growth potential, with countries like India and South Korea rapidly adopting aesthetic technologies and witnessing an increasing number of procedures. Economic policies supporting healthcare infrastructure and rising disposable incomes are key drivers in these emerging markets.
Asia-Pacific Medical Aesthetic Devices Industry Product Developments
Product innovation is a cornerstone of the Asia-Pacific medical aesthetic devices market. Manufacturers are continuously introducing advanced laser-based aesthetic devices with enhanced precision and targeted wavelengths for skin resurfacing and tattoo removal. The development of novel dermal fillers and Botulinum Toxin formulations with improved longevity and natural-looking results addresses the growing demand for minimally invasive treatments. Furthermore, the emergence of sophisticated RF-based aesthetic devices and ultrasound aesthetic devices for body contouring and skin tightening highlights a commitment to non-surgical solutions. These developments are driven by a focus on patient safety, efficacy, and reduced recovery times, allowing companies to gain a competitive edge by offering cutting-edge aesthetic technologies.
Key Drivers of Asia-Pacific Medical Aesthetic Devices Industry Growth
The Asia-Pacific medical aesthetic devices industry is experiencing robust growth fueled by several key drivers. Increasing disposable incomes across the region enable a larger segment of the population to afford elective aesthetic procedures. A significant surge in consumer awareness and acceptance of medical aesthetics plays a pivotal role, driven by media influence and a desire for enhanced appearance. Technological advancements in devices, leading to greater efficacy, safety, and less invasive procedures, are attracting more users. Furthermore, favorable regulatory environments in some key markets, coupled with government initiatives supporting healthcare innovation, also contribute to market expansion.
Challenges in the Asia-Pacific Medical Aesthetic Devices Industry Market
Despite its promising growth trajectory, the Asia-Pacific medical aesthetic devices market faces several challenges. Stringent regulatory hurdles in certain countries can delay product approvals and market entry, increasing development costs. High initial investment costs for advanced medical aesthetic devices can be a barrier for smaller clinics and emerging markets. Intense price competition among manufacturers and service providers can impact profit margins. Moreover, ethical concerns and the potential for misuse of certain devices necessitate continuous vigilance and education. Addressing these challenges will be crucial for sustained and responsible market growth.
Emerging Opportunities in Asia-Pacific Medical Aesthetic Devices Industry
The Asia-Pacific medical aesthetic devices industry is ripe with emerging opportunities for growth and innovation. The burgeoning demand for non-surgical and minimally invasive treatments continues to drive innovation in areas like dermal fillers, Botulinum Toxin, and advanced RF-based aesthetic devices. The increasing adoption of home-use aesthetic devices presents a significant untapped market potential, especially for facial rejuvenation and hair removal. Strategic partnerships between technology providers and healthcare institutions can accelerate the adoption of advanced devices. Furthermore, the expanding middle class in countries like India and Southeast Asian nations offers immense scope for market penetration and customized product offerings.
Leading Players in the Asia-Pacific Medical Aesthetic Devices Industry Sector
- Sciton Inc
- Candela Corporation
- Hologic Inc
- Sanuwave Health Inc
- LUTRONIC
- Boston Scientific Inc (Lumenis Inc )
- WON TECH Co Ltd
- Alma Lasers
- Cutera Inc
- Venus Concept
- Galderma SA (Nestle)
- AbbVie (Allergan PLC)
- Bausch Health Companies Inc
Key Milestones in Asia-Pacific Medical Aesthetic Devices Industry Industry
- August 2022: Allergan Healthcare India Private Limited launched Juvéderm VOLUX, a combination of Hyaluronic acid 25 mg + Lidocaine hydrochloride 3 mg, under its Business Unit of Allergan Aesthetics in India. This injectable implant is designed to restore and create volume in the face, marking a significant advancement in facial augmentation.
- July 2022: CurrentBody, Australia, launched Lip Perfector. This device, featuring 56 LED lights and four wavelengths, is designed to rejuvenate lips by boosting circulation, reducing redness, and stimulating collagen production for plumper, firmer lips, highlighting innovation in at-home aesthetic devices.
Strategic Outlook for Asia-Pacific Medical Aesthetic Devices Industry Market
The strategic outlook for the Asia-Pacific medical aesthetic devices market is overwhelmingly positive, driven by sustained consumer interest and ongoing technological advancements. The future will likely see a greater emphasis on personalized treatments and combination therapies, integrating energy-based and non-energy-based devices for optimal results. Expansion into tier-2 and tier-3 cities across the region presents a substantial growth accelerator. Companies that focus on developing user-friendly, cost-effective, and technologically sophisticated devices, while navigating evolving regulatory landscapes, will be well-positioned to capture significant market share and drive the industry forward. The increasing integration of digital technologies for patient consultation and follow-up will also be a key strategic imperative.
Asia-Pacific Medical Aesthetic Devices Industry Segmentation
-
1. Type of Device
-
1.1. Energy-based Aesthetic Device
- 1.1.1. Laser-based Aesthetic Device
- 1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 1.1.3. Light-based Aesthetic Device
- 1.1.4. Ultrasound Aesthetic Device
-
1.2. Non-energy-based Aesthetic Device
- 1.2.1. Botulinum Toxin
- 1.2.2. Dermal Fillers and Aesthetic Threads
- 1.2.3. Chemical Peels
- 1.2.4. Microdermabrasion
-
1.2.5. Implants
- 1.2.5.1. Facial Implants
- 1.2.5.2. Breast Implants
- 1.2.5.3. Other Implants
- 1.3. Other Non-energy-based Aesthetic Devices
-
1.1. Energy-based Aesthetic Device
-
2. Application
- 2.1. Skin Resurfacing and Tightening
- 2.2. Body Contouring and Cellulite Reduction
- 2.3. Hair Removal
- 2.4. Tattoo Removal
- 2.5. Breast Augmentation
- 2.6. Other Applications
-
3. End User
- 3.1. Hospitals
- 3.2. Clinics
- 3.3. Home settings
-
4. Geography
- 4.1. China
- 4.2. Japan
- 4.3. India
- 4.4. Australia
- 4.5. South Korea
- 4.6. Rest of Asia-Pacific
Asia-Pacific Medical Aesthetic Devices Industry Segmentation By Geography
- 1. China
- 2. Japan
- 3. India
- 4. Australia
- 5. South Korea
- 6. Rest of Asia Pacific
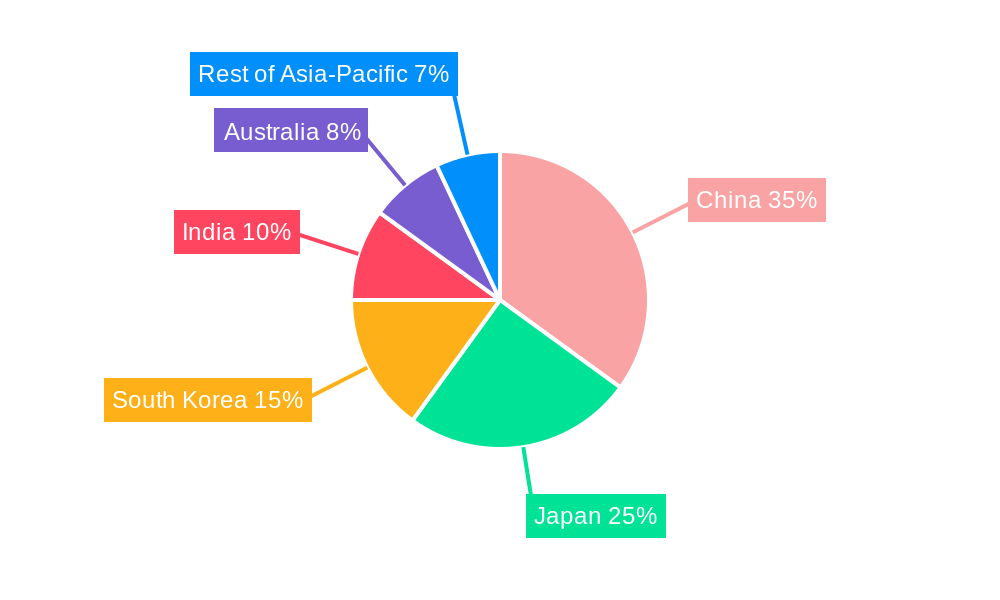
Asia-Pacific Medical Aesthetic Devices Industry Regional Market Share
Geographic Coverage of Asia-Pacific Medical Aesthetic Devices Industry
Asia-Pacific Medical Aesthetic Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 13.8% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Technological Advancement in Devices; Rise in Medical Tourism in Asian Countries; Increasing Obese Population
- 3.3. Market Restrains
- 3.3.1. Social Stigma Concerns; Poor Reimbursement Scenario
- 3.4. Market Trends
- 3.4.1. Dermal Fillers and Aesthetic Threads are Expected to Register a High Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 5.1.1. Energy-based Aesthetic Device
- 5.1.1.1. Laser-based Aesthetic Device
- 5.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 5.1.1.3. Light-based Aesthetic Device
- 5.1.1.4. Ultrasound Aesthetic Device
- 5.1.2. Non-energy-based Aesthetic Device
- 5.1.2.1. Botulinum Toxin
- 5.1.2.2. Dermal Fillers and Aesthetic Threads
- 5.1.2.3. Chemical Peels
- 5.1.2.4. Microdermabrasion
- 5.1.2.5. Implants
- 5.1.2.5.1. Facial Implants
- 5.1.2.5.2. Breast Implants
- 5.1.2.5.3. Other Implants
- 5.1.3. Other Non-energy-based Aesthetic Devices
- 5.1.1. Energy-based Aesthetic Device
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Skin Resurfacing and Tightening
- 5.2.2. Body Contouring and Cellulite Reduction
- 5.2.3. Hair Removal
- 5.2.4. Tattoo Removal
- 5.2.5. Breast Augmentation
- 5.2.6. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by End User
- 5.3.1. Hospitals
- 5.3.2. Clinics
- 5.3.3. Home settings
- 5.4. Market Analysis, Insights and Forecast - by Geography
- 5.4.1. China
- 5.4.2. Japan
- 5.4.3. India
- 5.4.4. Australia
- 5.4.5. South Korea
- 5.4.6. Rest of Asia-Pacific
- 5.5. Market Analysis, Insights and Forecast - by Region
- 5.5.1. China
- 5.5.2. Japan
- 5.5.3. India
- 5.5.4. Australia
- 5.5.5. South Korea
- 5.5.6. Rest of Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 6. China Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Type of Device
- 6.1.1. Energy-based Aesthetic Device
- 6.1.1.1. Laser-based Aesthetic Device
- 6.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 6.1.1.3. Light-based Aesthetic Device
- 6.1.1.4. Ultrasound Aesthetic Device
- 6.1.2. Non-energy-based Aesthetic Device
- 6.1.2.1. Botulinum Toxin
- 6.1.2.2. Dermal Fillers and Aesthetic Threads
- 6.1.2.3. Chemical Peels
- 6.1.2.4. Microdermabrasion
- 6.1.2.5. Implants
- 6.1.2.5.1. Facial Implants
- 6.1.2.5.2. Breast Implants
- 6.1.2.5.3. Other Implants
- 6.1.3. Other Non-energy-based Aesthetic Devices
- 6.1.1. Energy-based Aesthetic Device
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Skin Resurfacing and Tightening
- 6.2.2. Body Contouring and Cellulite Reduction
- 6.2.3. Hair Removal
- 6.2.4. Tattoo Removal
- 6.2.5. Breast Augmentation
- 6.2.6. Other Applications
- 6.3. Market Analysis, Insights and Forecast - by End User
- 6.3.1. Hospitals
- 6.3.2. Clinics
- 6.3.3. Home settings
- 6.4. Market Analysis, Insights and Forecast - by Geography
- 6.4.1. China
- 6.4.2. Japan
- 6.4.3. India
- 6.4.4. Australia
- 6.4.5. South Korea
- 6.4.6. Rest of Asia-Pacific
- 6.1. Market Analysis, Insights and Forecast - by Type of Device
- 7. Japan Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Type of Device
- 7.1.1. Energy-based Aesthetic Device
- 7.1.1.1. Laser-based Aesthetic Device
- 7.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 7.1.1.3. Light-based Aesthetic Device
- 7.1.1.4. Ultrasound Aesthetic Device
- 7.1.2. Non-energy-based Aesthetic Device
- 7.1.2.1. Botulinum Toxin
- 7.1.2.2. Dermal Fillers and Aesthetic Threads
- 7.1.2.3. Chemical Peels
- 7.1.2.4. Microdermabrasion
- 7.1.2.5. Implants
- 7.1.2.5.1. Facial Implants
- 7.1.2.5.2. Breast Implants
- 7.1.2.5.3. Other Implants
- 7.1.3. Other Non-energy-based Aesthetic Devices
- 7.1.1. Energy-based Aesthetic Device
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Skin Resurfacing and Tightening
- 7.2.2. Body Contouring and Cellulite Reduction
- 7.2.3. Hair Removal
- 7.2.4. Tattoo Removal
- 7.2.5. Breast Augmentation
- 7.2.6. Other Applications
- 7.3. Market Analysis, Insights and Forecast - by End User
- 7.3.1. Hospitals
- 7.3.2. Clinics
- 7.3.3. Home settings
- 7.4. Market Analysis, Insights and Forecast - by Geography
- 7.4.1. China
- 7.4.2. Japan
- 7.4.3. India
- 7.4.4. Australia
- 7.4.5. South Korea
- 7.4.6. Rest of Asia-Pacific
- 7.1. Market Analysis, Insights and Forecast - by Type of Device
- 8. India Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Type of Device
- 8.1.1. Energy-based Aesthetic Device
- 8.1.1.1. Laser-based Aesthetic Device
- 8.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 8.1.1.3. Light-based Aesthetic Device
- 8.1.1.4. Ultrasound Aesthetic Device
- 8.1.2. Non-energy-based Aesthetic Device
- 8.1.2.1. Botulinum Toxin
- 8.1.2.2. Dermal Fillers and Aesthetic Threads
- 8.1.2.3. Chemical Peels
- 8.1.2.4. Microdermabrasion
- 8.1.2.5. Implants
- 8.1.2.5.1. Facial Implants
- 8.1.2.5.2. Breast Implants
- 8.1.2.5.3. Other Implants
- 8.1.3. Other Non-energy-based Aesthetic Devices
- 8.1.1. Energy-based Aesthetic Device
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Skin Resurfacing and Tightening
- 8.2.2. Body Contouring and Cellulite Reduction
- 8.2.3. Hair Removal
- 8.2.4. Tattoo Removal
- 8.2.5. Breast Augmentation
- 8.2.6. Other Applications
- 8.3. Market Analysis, Insights and Forecast - by End User
- 8.3.1. Hospitals
- 8.3.2. Clinics
- 8.3.3. Home settings
- 8.4. Market Analysis, Insights and Forecast - by Geography
- 8.4.1. China
- 8.4.2. Japan
- 8.4.3. India
- 8.4.4. Australia
- 8.4.5. South Korea
- 8.4.6. Rest of Asia-Pacific
- 8.1. Market Analysis, Insights and Forecast - by Type of Device
- 9. Australia Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Type of Device
- 9.1.1. Energy-based Aesthetic Device
- 9.1.1.1. Laser-based Aesthetic Device
- 9.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 9.1.1.3. Light-based Aesthetic Device
- 9.1.1.4. Ultrasound Aesthetic Device
- 9.1.2. Non-energy-based Aesthetic Device
- 9.1.2.1. Botulinum Toxin
- 9.1.2.2. Dermal Fillers and Aesthetic Threads
- 9.1.2.3. Chemical Peels
- 9.1.2.4. Microdermabrasion
- 9.1.2.5. Implants
- 9.1.2.5.1. Facial Implants
- 9.1.2.5.2. Breast Implants
- 9.1.2.5.3. Other Implants
- 9.1.3. Other Non-energy-based Aesthetic Devices
- 9.1.1. Energy-based Aesthetic Device
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Skin Resurfacing and Tightening
- 9.2.2. Body Contouring and Cellulite Reduction
- 9.2.3. Hair Removal
- 9.2.4. Tattoo Removal
- 9.2.5. Breast Augmentation
- 9.2.6. Other Applications
- 9.3. Market Analysis, Insights and Forecast - by End User
- 9.3.1. Hospitals
- 9.3.2. Clinics
- 9.3.3. Home settings
- 9.4. Market Analysis, Insights and Forecast - by Geography
- 9.4.1. China
- 9.4.2. Japan
- 9.4.3. India
- 9.4.4. Australia
- 9.4.5. South Korea
- 9.4.6. Rest of Asia-Pacific
- 9.1. Market Analysis, Insights and Forecast - by Type of Device
- 10. South Korea Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Type of Device
- 10.1.1. Energy-based Aesthetic Device
- 10.1.1.1. Laser-based Aesthetic Device
- 10.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 10.1.1.3. Light-based Aesthetic Device
- 10.1.1.4. Ultrasound Aesthetic Device
- 10.1.2. Non-energy-based Aesthetic Device
- 10.1.2.1. Botulinum Toxin
- 10.1.2.2. Dermal Fillers and Aesthetic Threads
- 10.1.2.3. Chemical Peels
- 10.1.2.4. Microdermabrasion
- 10.1.2.5. Implants
- 10.1.2.5.1. Facial Implants
- 10.1.2.5.2. Breast Implants
- 10.1.2.5.3. Other Implants
- 10.1.3. Other Non-energy-based Aesthetic Devices
- 10.1.1. Energy-based Aesthetic Device
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Skin Resurfacing and Tightening
- 10.2.2. Body Contouring and Cellulite Reduction
- 10.2.3. Hair Removal
- 10.2.4. Tattoo Removal
- 10.2.5. Breast Augmentation
- 10.2.6. Other Applications
- 10.3. Market Analysis, Insights and Forecast - by End User
- 10.3.1. Hospitals
- 10.3.2. Clinics
- 10.3.3. Home settings
- 10.4. Market Analysis, Insights and Forecast - by Geography
- 10.4.1. China
- 10.4.2. Japan
- 10.4.3. India
- 10.4.4. Australia
- 10.4.5. South Korea
- 10.4.6. Rest of Asia-Pacific
- 10.1. Market Analysis, Insights and Forecast - by Type of Device
- 11. Rest of Asia Pacific Asia-Pacific Medical Aesthetic Devices Industry Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Type of Device
- 11.1.1. Energy-based Aesthetic Device
- 11.1.1.1. Laser-based Aesthetic Device
- 11.1.1.2. Radiofrequency (RF)-based Aesthetic Device
- 11.1.1.3. Light-based Aesthetic Device
- 11.1.1.4. Ultrasound Aesthetic Device
- 11.1.2. Non-energy-based Aesthetic Device
- 11.1.2.1. Botulinum Toxin
- 11.1.2.2. Dermal Fillers and Aesthetic Threads
- 11.1.2.3. Chemical Peels
- 11.1.2.4. Microdermabrasion
- 11.1.2.5. Implants
- 11.1.2.5.1. Facial Implants
- 11.1.2.5.2. Breast Implants
- 11.1.2.5.3. Other Implants
- 11.1.3. Other Non-energy-based Aesthetic Devices
- 11.1.1. Energy-based Aesthetic Device
- 11.2. Market Analysis, Insights and Forecast - by Application
- 11.2.1. Skin Resurfacing and Tightening
- 11.2.2. Body Contouring and Cellulite Reduction
- 11.2.3. Hair Removal
- 11.2.4. Tattoo Removal
- 11.2.5. Breast Augmentation
- 11.2.6. Other Applications
- 11.3. Market Analysis, Insights and Forecast - by End User
- 11.3.1. Hospitals
- 11.3.2. Clinics
- 11.3.3. Home settings
- 11.4. Market Analysis, Insights and Forecast - by Geography
- 11.4.1. China
- 11.4.2. Japan
- 11.4.3. India
- 11.4.4. Australia
- 11.4.5. South Korea
- 11.4.6. Rest of Asia-Pacific
- 11.1. Market Analysis, Insights and Forecast - by Type of Device
- 12. Competitive Analysis
- 12.1. Market Share Analysis 2025
- 12.2. Company Profiles
- 12.2.1 Sciton Inc *List Not Exhaustive
- 12.2.1.1. Overview
- 12.2.1.2. Products
- 12.2.1.3. SWOT Analysis
- 12.2.1.4. Recent Developments
- 12.2.1.5. Financials (Based on Availability)
- 12.2.2 Candela Corporation
- 12.2.2.1. Overview
- 12.2.2.2. Products
- 12.2.2.3. SWOT Analysis
- 12.2.2.4. Recent Developments
- 12.2.2.5. Financials (Based on Availability)
- 12.2.3 Hologic Inc
- 12.2.3.1. Overview
- 12.2.3.2. Products
- 12.2.3.3. SWOT Analysis
- 12.2.3.4. Recent Developments
- 12.2.3.5. Financials (Based on Availability)
- 12.2.4 Sanuwave Health Inc
- 12.2.4.1. Overview
- 12.2.4.2. Products
- 12.2.4.3. SWOT Analysis
- 12.2.4.4. Recent Developments
- 12.2.4.5. Financials (Based on Availability)
- 12.2.5 LUTRONIC
- 12.2.5.1. Overview
- 12.2.5.2. Products
- 12.2.5.3. SWOT Analysis
- 12.2.5.4. Recent Developments
- 12.2.5.5. Financials (Based on Availability)
- 12.2.6 Boston Scientific Inc (Lumenis Inc )
- 12.2.6.1. Overview
- 12.2.6.2. Products
- 12.2.6.3. SWOT Analysis
- 12.2.6.4. Recent Developments
- 12.2.6.5. Financials (Based on Availability)
- 12.2.7 WON TECH Co Ltd
- 12.2.7.1. Overview
- 12.2.7.2. Products
- 12.2.7.3. SWOT Analysis
- 12.2.7.4. Recent Developments
- 12.2.7.5. Financials (Based on Availability)
- 12.2.8 Alma Lasers
- 12.2.8.1. Overview
- 12.2.8.2. Products
- 12.2.8.3. SWOT Analysis
- 12.2.8.4. Recent Developments
- 12.2.8.5. Financials (Based on Availability)
- 12.2.9 Cutera Inc
- 12.2.9.1. Overview
- 12.2.9.2. Products
- 12.2.9.3. SWOT Analysis
- 12.2.9.4. Recent Developments
- 12.2.9.5. Financials (Based on Availability)
- 12.2.10 Venus Concept
- 12.2.10.1. Overview
- 12.2.10.2. Products
- 12.2.10.3. SWOT Analysis
- 12.2.10.4. Recent Developments
- 12.2.10.5. Financials (Based on Availability)
- 12.2.11 Galderma SA (Nestle)
- 12.2.11.1. Overview
- 12.2.11.2. Products
- 12.2.11.3. SWOT Analysis
- 12.2.11.4. Recent Developments
- 12.2.11.5. Financials (Based on Availability)
- 12.2.12 AbbVie (Allergan PLC)
- 12.2.12.1. Overview
- 12.2.12.2. Products
- 12.2.12.3. SWOT Analysis
- 12.2.12.4. Recent Developments
- 12.2.12.5. Financials (Based on Availability)
- 12.2.13 Bausch Health Companies Inc
- 12.2.13.1. Overview
- 12.2.13.2. Products
- 12.2.13.3. SWOT Analysis
- 12.2.13.4. Recent Developments
- 12.2.13.5. Financials (Based on Availability)
- 12.2.1 Sciton Inc *List Not Exhaustive
List of Figures
- Figure 1: Asia-Pacific Medical Aesthetic Devices Industry Revenue Breakdown (undefined, %) by Product 2025 & 2033
- Figure 2: Asia-Pacific Medical Aesthetic Devices Industry Share (%) by Company 2025
List of Tables
- Table 1: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 2: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 3: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 4: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 5: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Region 2020 & 2033
- Table 6: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 7: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 8: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 9: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 10: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 11: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 12: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 13: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 14: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 15: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 16: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 17: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 18: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 19: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 20: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 21: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 22: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 23: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 24: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 25: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 26: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 27: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 28: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 29: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 30: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Country 2020 & 2033
- Table 31: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Type of Device 2020 & 2033
- Table 32: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Application 2020 & 2033
- Table 33: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by End User 2020 & 2033
- Table 34: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Geography 2020 & 2033
- Table 35: Asia-Pacific Medical Aesthetic Devices Industry Revenue undefined Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Asia-Pacific Medical Aesthetic Devices Industry?
The projected CAGR is approximately 13.8%.
2. Which companies are prominent players in the Asia-Pacific Medical Aesthetic Devices Industry?
Key companies in the market include Sciton Inc *List Not Exhaustive, Candela Corporation, Hologic Inc, Sanuwave Health Inc, LUTRONIC, Boston Scientific Inc (Lumenis Inc ), WON TECH Co Ltd, Alma Lasers, Cutera Inc, Venus Concept, Galderma SA (Nestle), AbbVie (Allergan PLC), Bausch Health Companies Inc.
3. What are the main segments of the Asia-Pacific Medical Aesthetic Devices Industry?
The market segments include Type of Device, Application, End User, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
Technological Advancement in Devices; Rise in Medical Tourism in Asian Countries; Increasing Obese Population.
6. What are the notable trends driving market growth?
Dermal Fillers and Aesthetic Threads are Expected to Register a High Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
Social Stigma Concerns; Poor Reimbursement Scenario.
8. Can you provide examples of recent developments in the market?
In August 2022, Allergan Healthcare India Private Limited launched Juvéderm VOLUX, a combination of Hyaluronic acid 25 mg + Lidocaine hydrochloride 3 mg, under its Business Unit of Allergan Aesthetics in India. It is an injectable implant intended to restore and create volume in the face.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Asia-Pacific Medical Aesthetic Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Asia-Pacific Medical Aesthetic Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Asia-Pacific Medical Aesthetic Devices Industry?
To stay informed about further developments, trends, and reports in the Asia-Pacific Medical Aesthetic Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
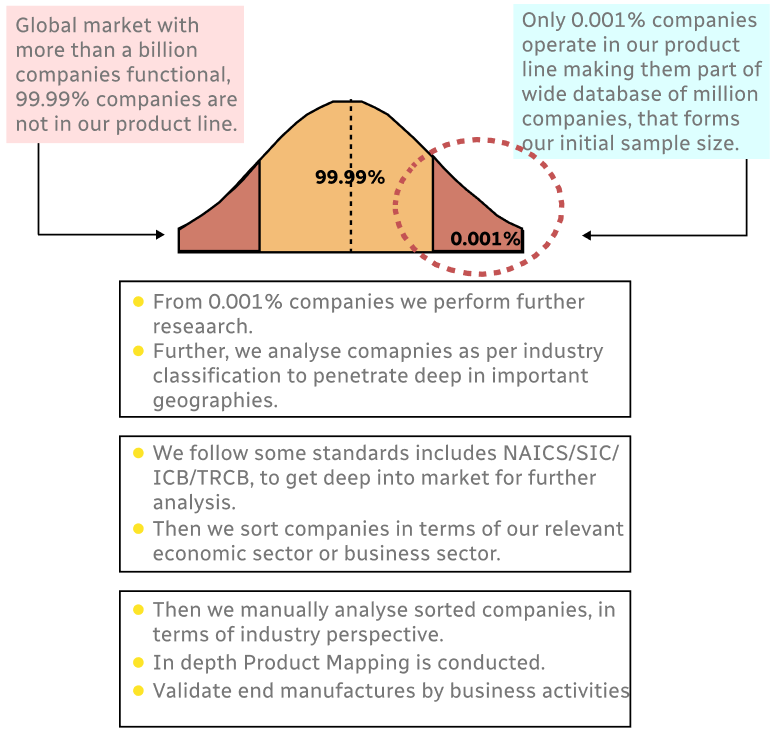
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


