Key Insights
The European cancer biological therapy market, valued at €58.32 billion in 2025, is projected to experience robust growth, driven by a rising prevalence of various cancer types, an aging population, and advancements in immunotherapy and targeted therapies. The market's Compound Annual Growth Rate (CAGR) of 5.38% from 2025 to 2033 indicates a significant expansion over the forecast period. Key growth drivers include increased healthcare expenditure, rising awareness about cancer treatment options, and the ongoing development of innovative biological therapies with improved efficacy and reduced side effects. The market is segmented by treatment type (chemotherapy, targeted therapy, immunotherapy, hormonal therapy, and others), cancer type (blood cancer, breast cancer, prostate cancer, gastrointestinal cancer, gynecological cancer, respiratory/lung cancer, and others), and end-user (hospitals, specialty clinics, and cancer/radiation therapy centers). Immunotherapy, particularly checkpoint inhibitors, is expected to witness substantial growth, owing to its targeted mechanism and improved patient outcomes. However, high treatment costs, stringent regulatory approvals, and potential side effects pose challenges to market expansion. Germany, France, the UK, and Italy represent major market segments within Europe, reflecting their established healthcare infrastructure and higher cancer incidence rates. The competitive landscape features major pharmaceutical companies actively involved in research and development, resulting in an ongoing influx of novel therapeutic agents and therapies for various cancer types.
Cancer Biological Therapy Industry in Europe Market Size (In Billion)
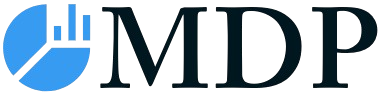
The market's segmentation offers further insight into specific growth areas. Blood cancers, due to their high prevalence and often aggressive nature, are expected to drive significant demand for biological therapies. Similarly, breast and lung cancers, owing to their high incidence rates across Europe, represent substantial market opportunities. The dominance of large pharmaceutical companies indicates high levels of competition and a focus on innovation within the industry. Future growth will largely depend on continued research and development efforts focused on improving treatment efficacy, reducing toxicity, and personalizing cancer care. Expansion into emerging markets within Europe and strategic partnerships could further fuel market growth. Increased government initiatives aimed at improving cancer care access will also play a crucial role in shaping the market’s trajectory.
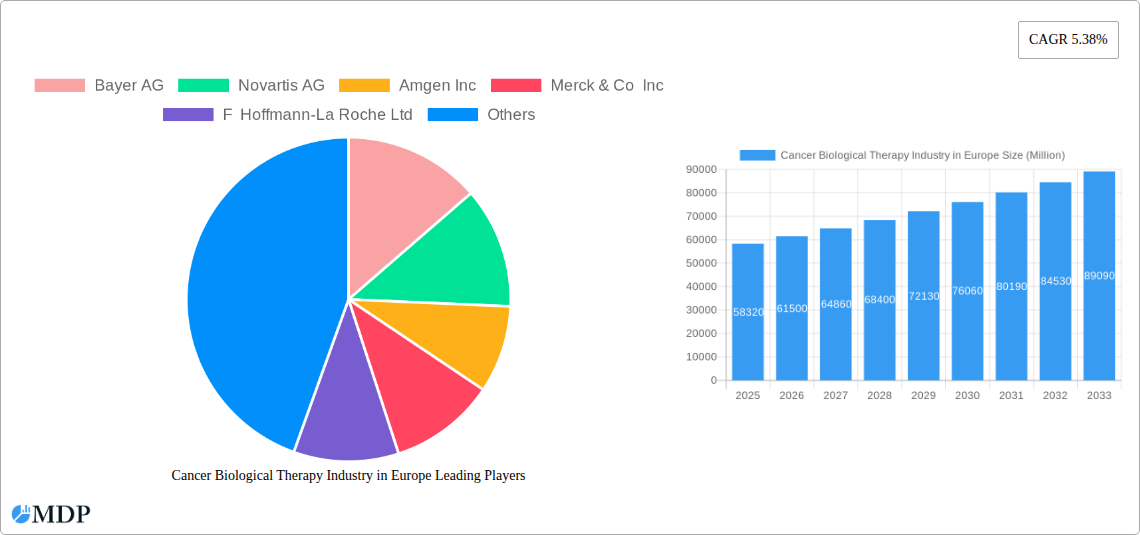
Cancer Biological Therapy Industry in Europe Company Market Share
Cancer Biological Therapy Industry in Europe: A Comprehensive Market Report (2019-2033)
This comprehensive report provides a detailed analysis of the Cancer Biological Therapy industry in Europe, covering market dynamics, trends, leading players, and future growth prospects. The report utilizes data from the historical period (2019-2024), base year (2025), and forecasts through 2033, offering invaluable insights for industry stakeholders, investors, and strategic decision-makers. The European market for cancer biological therapies is poised for significant growth, driven by technological advancements and increasing prevalence of various cancer types. This report offers a detailed breakdown by treatment type, cancer type, and end-user, enabling informed strategic planning.
Cancer Biological Therapy Industry in Europe Market Dynamics & Concentration
The European cancer biological therapy market is characterized by high concentration, with several multinational pharmaceutical giants holding significant market share. The top 10 players, including Bayer AG, Novartis AG, Amgen Inc, Merck & Co Inc, F Hoffmann-La Roche Ltd, AstraZeneca PLC, Pfizer Inc, Johnson & Johnson, Bristol-Myers Squibb Company, and GlaxoSmithKline PLC, collectively account for approximately 75% of the market. Market share fluctuates based on new drug approvals, clinical trial outcomes, and successful M&A activities. Innovation is a key driver, with continuous R&D efforts focused on developing novel therapies like targeted therapies and immunotherapies. The regulatory landscape, while stringent, is designed to ensure patient safety and efficacy, influencing product development and market entry strategies. The presence of substitute treatments (e.g., conventional chemotherapy) exerts competitive pressure, while growing end-user preference for targeted and less toxic therapies fuels market expansion.
- Market Concentration: High, with top 10 players controlling ~75% of market share (2025 estimate).
- M&A Activity: Significant, with an estimated xx deals in the 2019-2024 period, driven by portfolio expansion and technological access.
- Innovation Drivers: Investment in R&D for novel biologics, including CAR T-cell therapies and immune checkpoint inhibitors.
- Regulatory Framework: Stringent, influencing time to market and overall cost of development.
Cancer Biological Therapy Industry in Europe Industry Trends & Analysis
The European cancer biological therapy market exhibits a robust Compound Annual Growth Rate (CAGR) of xx% during the forecast period (2025-2033). This growth is fueled by several factors, including an aging population leading to increased cancer incidence, rising healthcare expenditure, and growing awareness among patients regarding advanced treatment options. Technological advancements in genomics and personalized medicine are leading to more targeted and effective therapies, improving patient outcomes and increasing market penetration. However, the market faces challenges such as high treatment costs, the need for specialized infrastructure and skilled healthcare professionals, and a complex regulatory approval process. Intense competition among established players and emerging biotech companies drives innovation, accelerates the pace of drug development, and shapes market dynamics. The market penetration of biological therapies is increasing steadily, with a projected xx% of the total cancer treatment market by 2033.
Leading Markets & Segments in Cancer Biological Therapy Industry in Europe
Germany, France, and the United Kingdom represent the largest markets for cancer biological therapies in Europe, driven by high healthcare spending, well-established healthcare infrastructure, and a relatively high prevalence of cancer. Within the treatment segments, Immunotherapy (Biologic Therapy) is experiencing the most significant growth, fueled by its targeted approach and enhanced efficacy. Among cancer types, Blood Cancer and Breast Cancer represent the largest segments due to high incidence rates and a broader range of available treatment options. Hospitals, specialty clinics, and cancer/radiation therapy centers are the primary end-users for cancer biological therapies.
- Key Drivers for Leading Markets:
- Germany: Strong healthcare infrastructure, high R&D investment, and large pharmaceutical presence.
- France: Significant public healthcare expenditure, high cancer incidence rates.
- United Kingdom: Well-developed healthcare system, high prevalence of certain cancer types.
- Dominant Segments: Immunotherapy (Biologic Therapy) showing highest growth; Blood Cancer and Breast Cancer largest by volume.
- End-User Dominance: Hospitals remain the largest end user, followed by specialty clinics and cancer treatment centers.
Cancer Biological Therapy Industry in Europe Product Developments
The cancer biological therapy market is witnessing continuous product innovation. Recent advancements include personalized cancer vaccines, next-generation CAR T-cell therapies, and improved antibody-drug conjugates. These innovations offer enhanced efficacy, reduced side effects, and targeted treatment approaches, improving patient outcomes and driving market expansion. The market also sees developments in combination therapies, utilizing multiple biological agents to synergistically target cancer cells. This approach demonstrates enhanced efficacy against various cancer types.
Key Drivers of Cancer Biological Therapy Industry in Europe Growth
Several factors drive the growth of the European cancer biological therapy market: increasing cancer prevalence due to an aging population, rising healthcare expenditure and insurance coverage, growing awareness of advanced treatment options among patients and physicians, continuous technological innovation, leading to more effective and targeted therapies, increasing research and development efforts by pharmaceutical and biotechnology companies. Government initiatives and regulatory support for the development and adoption of new cancer therapies, and finally, increasing strategic alliances and collaborations between pharmaceutical companies and research institutions further fuel market growth.
Challenges in the Cancer Biological Therapy Industry in Europe Market
The European cancer biological therapy market faces challenges, including high drug prices that limit accessibility for some patients, complex regulatory approval processes that can delay product launches, and logistical hurdles related to the cold chain management of many biological products. The emergence of biosimilars introduces price competition, potentially affecting profitability, while ensuring access to affordable therapies. The intense competition among established players and emerging biotech companies creates a highly dynamic market environment.
Emerging Opportunities in Cancer Biological Therapy Industry in Europe
Significant opportunities exist in the European cancer biological therapy market. These include the development of next-generation immunotherapy approaches, such as oncolytic viruses and bispecific antibodies, and personalized medicine strategies tailored to individual patients' genetic profiles. The market is ripe for expansion in less developed European markets with significant unmet medical needs. Strategic partnerships and collaborations between pharmaceutical companies and academic research institutions can facilitate the discovery and development of innovative cancer therapies.
Leading Players in the Cancer Biological Therapy Industry in Europe Sector
Key Milestones in Cancer Biological Therapy Industry in Europe Industry
- May 2022: Boehringer Ingelheim acquired Northern Biologics, expanding its cancer immunology portfolio.
- June 2021: Genentech collaborated with Nykode to develop novel DNA-based cancer vaccines.
Strategic Outlook for Cancer Biological Therapy Industry in Europe Market
The European cancer biological therapy market is projected to experience significant growth over the forecast period, driven by factors such as increasing prevalence of cancer, technological advancements, and supportive regulatory frameworks. Strategic opportunities exist in developing innovative therapies, expanding into new markets, and forging strategic alliances. Companies focusing on personalized medicine approaches, combination therapies, and overcoming manufacturing and distribution challenges are poised for success. The market will continue to be highly dynamic, driven by innovation and intense competition among leading players.
Cancer Biological Therapy Industry in Europe Segmentation
-
1. Treatment
- 1.1. Chemotherapy
- 1.2. Target Therapy
- 1.3. Immunotherapy (Biologic Therapy)
- 1.4. Hormonal Therapy
- 1.5. Other Treatment Types
-
2. Cancer Type
- 2.1. Blood Cancer
- 2.2. Breast Cancer
- 2.3. Prostate Cancer
- 2.4. Gastrointestinal Cancer
- 2.5. Gynecologic Cancer
- 2.6. Respiratory/Lung Cancer
- 2.7. Other Cancer Types
-
3. End User
- 3.1. Hospitals
- 3.2. Specilty Clinics
- 3.3. Cancer and Radiation Therapy Centers
Cancer Biological Therapy Industry in Europe Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
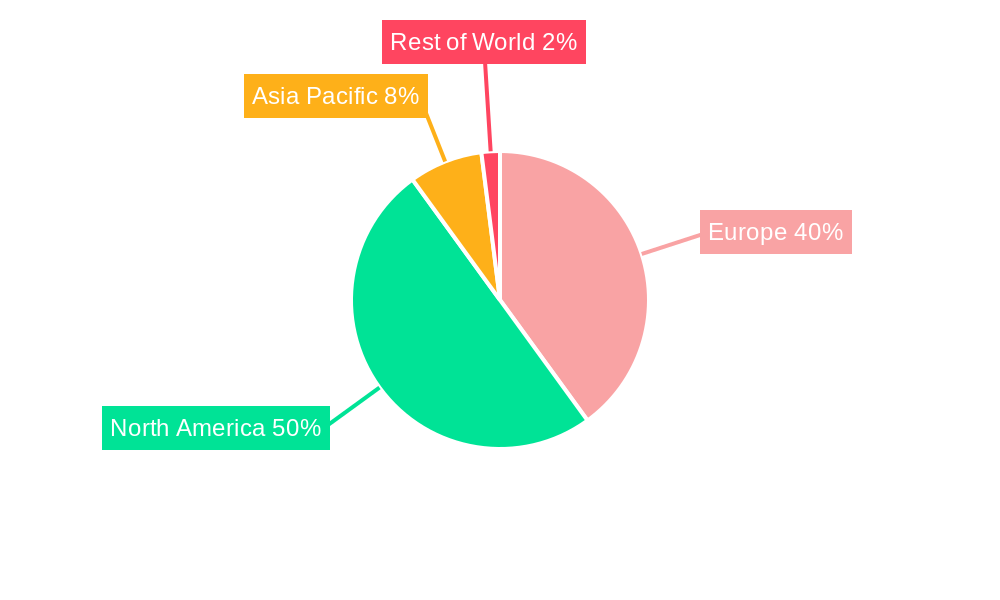
Cancer Biological Therapy Industry in Europe Regional Market Share
Geographic Coverage of Cancer Biological Therapy Industry in Europe
Cancer Biological Therapy Industry in Europe REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.38% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Prevalence of Cancer in Europe; Strong R&D Initiatives from Key Players
- 3.3. Market Restrains
- 3.3.1. Inequality in Access of Cancer Therapy across Europe; High Cost of Cancer Therapies
- 3.4. Market Trends
- 3.4.1. Target Therapy is Expected to Witness Significant Growth over the Forecast Period (2022-2027)
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Treatment
- 5.1.1. Chemotherapy
- 5.1.2. Target Therapy
- 5.1.3. Immunotherapy (Biologic Therapy)
- 5.1.4. Hormonal Therapy
- 5.1.5. Other Treatment Types
- 5.2. Market Analysis, Insights and Forecast - by Cancer Type
- 5.2.1. Blood Cancer
- 5.2.2. Breast Cancer
- 5.2.3. Prostate Cancer
- 5.2.4. Gastrointestinal Cancer
- 5.2.5. Gynecologic Cancer
- 5.2.6. Respiratory/Lung Cancer
- 5.2.7. Other Cancer Types
- 5.3. Market Analysis, Insights and Forecast - by End User
- 5.3.1. Hospitals
- 5.3.2. Specilty Clinics
- 5.3.3. Cancer and Radiation Therapy Centers
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. Germany
- 5.4.2. United Kingdom
- 5.4.3. France
- 5.4.4. Italy
- 5.4.5. Spain
- 5.4.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Treatment
- 6. Germany Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Treatment
- 6.1.1. Chemotherapy
- 6.1.2. Target Therapy
- 6.1.3. Immunotherapy (Biologic Therapy)
- 6.1.4. Hormonal Therapy
- 6.1.5. Other Treatment Types
- 6.2. Market Analysis, Insights and Forecast - by Cancer Type
- 6.2.1. Blood Cancer
- 6.2.2. Breast Cancer
- 6.2.3. Prostate Cancer
- 6.2.4. Gastrointestinal Cancer
- 6.2.5. Gynecologic Cancer
- 6.2.6. Respiratory/Lung Cancer
- 6.2.7. Other Cancer Types
- 6.3. Market Analysis, Insights and Forecast - by End User
- 6.3.1. Hospitals
- 6.3.2. Specilty Clinics
- 6.3.3. Cancer and Radiation Therapy Centers
- 6.1. Market Analysis, Insights and Forecast - by Treatment
- 7. United Kingdom Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Treatment
- 7.1.1. Chemotherapy
- 7.1.2. Target Therapy
- 7.1.3. Immunotherapy (Biologic Therapy)
- 7.1.4. Hormonal Therapy
- 7.1.5. Other Treatment Types
- 7.2. Market Analysis, Insights and Forecast - by Cancer Type
- 7.2.1. Blood Cancer
- 7.2.2. Breast Cancer
- 7.2.3. Prostate Cancer
- 7.2.4. Gastrointestinal Cancer
- 7.2.5. Gynecologic Cancer
- 7.2.6. Respiratory/Lung Cancer
- 7.2.7. Other Cancer Types
- 7.3. Market Analysis, Insights and Forecast - by End User
- 7.3.1. Hospitals
- 7.3.2. Specilty Clinics
- 7.3.3. Cancer and Radiation Therapy Centers
- 7.1. Market Analysis, Insights and Forecast - by Treatment
- 8. France Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Treatment
- 8.1.1. Chemotherapy
- 8.1.2. Target Therapy
- 8.1.3. Immunotherapy (Biologic Therapy)
- 8.1.4. Hormonal Therapy
- 8.1.5. Other Treatment Types
- 8.2. Market Analysis, Insights and Forecast - by Cancer Type
- 8.2.1. Blood Cancer
- 8.2.2. Breast Cancer
- 8.2.3. Prostate Cancer
- 8.2.4. Gastrointestinal Cancer
- 8.2.5. Gynecologic Cancer
- 8.2.6. Respiratory/Lung Cancer
- 8.2.7. Other Cancer Types
- 8.3. Market Analysis, Insights and Forecast - by End User
- 8.3.1. Hospitals
- 8.3.2. Specilty Clinics
- 8.3.3. Cancer and Radiation Therapy Centers
- 8.1. Market Analysis, Insights and Forecast - by Treatment
- 9. Italy Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Treatment
- 9.1.1. Chemotherapy
- 9.1.2. Target Therapy
- 9.1.3. Immunotherapy (Biologic Therapy)
- 9.1.4. Hormonal Therapy
- 9.1.5. Other Treatment Types
- 9.2. Market Analysis, Insights and Forecast - by Cancer Type
- 9.2.1. Blood Cancer
- 9.2.2. Breast Cancer
- 9.2.3. Prostate Cancer
- 9.2.4. Gastrointestinal Cancer
- 9.2.5. Gynecologic Cancer
- 9.2.6. Respiratory/Lung Cancer
- 9.2.7. Other Cancer Types
- 9.3. Market Analysis, Insights and Forecast - by End User
- 9.3.1. Hospitals
- 9.3.2. Specilty Clinics
- 9.3.3. Cancer and Radiation Therapy Centers
- 9.1. Market Analysis, Insights and Forecast - by Treatment
- 10. Spain Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Treatment
- 10.1.1. Chemotherapy
- 10.1.2. Target Therapy
- 10.1.3. Immunotherapy (Biologic Therapy)
- 10.1.4. Hormonal Therapy
- 10.1.5. Other Treatment Types
- 10.2. Market Analysis, Insights and Forecast - by Cancer Type
- 10.2.1. Blood Cancer
- 10.2.2. Breast Cancer
- 10.2.3. Prostate Cancer
- 10.2.4. Gastrointestinal Cancer
- 10.2.5. Gynecologic Cancer
- 10.2.6. Respiratory/Lung Cancer
- 10.2.7. Other Cancer Types
- 10.3. Market Analysis, Insights and Forecast - by End User
- 10.3.1. Hospitals
- 10.3.2. Specilty Clinics
- 10.3.3. Cancer and Radiation Therapy Centers
- 10.1. Market Analysis, Insights and Forecast - by Treatment
- 11. Rest of Europe Cancer Biological Therapy Industry in Europe Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Treatment
- 11.1.1. Chemotherapy
- 11.1.2. Target Therapy
- 11.1.3. Immunotherapy (Biologic Therapy)
- 11.1.4. Hormonal Therapy
- 11.1.5. Other Treatment Types
- 11.2. Market Analysis, Insights and Forecast - by Cancer Type
- 11.2.1. Blood Cancer
- 11.2.2. Breast Cancer
- 11.2.3. Prostate Cancer
- 11.2.4. Gastrointestinal Cancer
- 11.2.5. Gynecologic Cancer
- 11.2.6. Respiratory/Lung Cancer
- 11.2.7. Other Cancer Types
- 11.3. Market Analysis, Insights and Forecast - by End User
- 11.3.1. Hospitals
- 11.3.2. Specilty Clinics
- 11.3.3. Cancer and Radiation Therapy Centers
- 11.1. Market Analysis, Insights and Forecast - by Treatment
- 12. Competitive Analysis
- 12.1. Market Share Analysis 2025
- 12.2. Company Profiles
- 12.2.1 Bayer AG
- 12.2.1.1. Overview
- 12.2.1.2. Products
- 12.2.1.3. SWOT Analysis
- 12.2.1.4. Recent Developments
- 12.2.1.5. Financials (Based on Availability)
- 12.2.2 Novartis AG
- 12.2.2.1. Overview
- 12.2.2.2. Products
- 12.2.2.3. SWOT Analysis
- 12.2.2.4. Recent Developments
- 12.2.2.5. Financials (Based on Availability)
- 12.2.3 Amgen Inc
- 12.2.3.1. Overview
- 12.2.3.2. Products
- 12.2.3.3. SWOT Analysis
- 12.2.3.4. Recent Developments
- 12.2.3.5. Financials (Based on Availability)
- 12.2.4 Merck & Co Inc
- 12.2.4.1. Overview
- 12.2.4.2. Products
- 12.2.4.3. SWOT Analysis
- 12.2.4.4. Recent Developments
- 12.2.4.5. Financials (Based on Availability)
- 12.2.5 F Hoffmann-La Roche Ltd
- 12.2.5.1. Overview
- 12.2.5.2. Products
- 12.2.5.3. SWOT Analysis
- 12.2.5.4. Recent Developments
- 12.2.5.5. Financials (Based on Availability)
- 12.2.6 AstraZeneca PLC
- 12.2.6.1. Overview
- 12.2.6.2. Products
- 12.2.6.3. SWOT Analysis
- 12.2.6.4. Recent Developments
- 12.2.6.5. Financials (Based on Availability)
- 12.2.7 Pfizer Inc
- 12.2.7.1. Overview
- 12.2.7.2. Products
- 12.2.7.3. SWOT Analysis
- 12.2.7.4. Recent Developments
- 12.2.7.5. Financials (Based on Availability)
- 12.2.8 Johnson & Johnson
- 12.2.8.1. Overview
- 12.2.8.2. Products
- 12.2.8.3. SWOT Analysis
- 12.2.8.4. Recent Developments
- 12.2.8.5. Financials (Based on Availability)
- 12.2.9 Bristol-Myers Squibb Company
- 12.2.9.1. Overview
- 12.2.9.2. Products
- 12.2.9.3. SWOT Analysis
- 12.2.9.4. Recent Developments
- 12.2.9.5. Financials (Based on Availability)
- 12.2.10 GlaxoSmithKline PLC
- 12.2.10.1. Overview
- 12.2.10.2. Products
- 12.2.10.3. SWOT Analysis
- 12.2.10.4. Recent Developments
- 12.2.10.5. Financials (Based on Availability)
- 12.2.1 Bayer AG
List of Figures
- Figure 1: Cancer Biological Therapy Industry in Europe Revenue Breakdown (Million, %) by Product 2025 & 2033
- Figure 2: Cancer Biological Therapy Industry in Europe Share (%) by Company 2025
List of Tables
- Table 1: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 2: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 3: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 4: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 5: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 6: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 7: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Region 2020 & 2033
- Table 8: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Region 2020 & 2033
- Table 9: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 10: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 11: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 12: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 13: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 14: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 15: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Country 2020 & 2033
- Table 16: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Country 2020 & 2033
- Table 17: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 18: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 19: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 20: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 21: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 22: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 23: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Country 2020 & 2033
- Table 24: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Country 2020 & 2033
- Table 25: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 26: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 27: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 28: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 29: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 30: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 31: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Country 2020 & 2033
- Table 32: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Country 2020 & 2033
- Table 33: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 34: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 35: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 36: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 37: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 38: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 39: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Country 2020 & 2033
- Table 40: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Country 2020 & 2033
- Table 41: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 42: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 43: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 44: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 45: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 46: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 47: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Country 2020 & 2033
- Table 48: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Country 2020 & 2033
- Table 49: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Treatment 2020 & 2033
- Table 50: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Treatment 2020 & 2033
- Table 51: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Cancer Type 2020 & 2033
- Table 52: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Cancer Type 2020 & 2033
- Table 53: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by End User 2020 & 2033
- Table 54: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by End User 2020 & 2033
- Table 55: Cancer Biological Therapy Industry in Europe Revenue Million Forecast, by Country 2020 & 2033
- Table 56: Cancer Biological Therapy Industry in Europe Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Cancer Biological Therapy Industry in Europe?
The projected CAGR is approximately 5.38%.
2. Which companies are prominent players in the Cancer Biological Therapy Industry in Europe?
Key companies in the market include Bayer AG, Novartis AG, Amgen Inc, Merck & Co Inc, F Hoffmann-La Roche Ltd, AstraZeneca PLC, Pfizer Inc , Johnson & Johnson, Bristol-Myers Squibb Company, GlaxoSmithKline PLC.
3. What are the main segments of the Cancer Biological Therapy Industry in Europe?
The market segments include Treatment, Cancer Type, End User.
4. Can you provide details about the market size?
The market size is estimated to be USD 58.32 Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Prevalence of Cancer in Europe; Strong R&D Initiatives from Key Players.
6. What are the notable trends driving market growth?
Target Therapy is Expected to Witness Significant Growth over the Forecast Period (2022-2027).
7. Are there any restraints impacting market growth?
Inequality in Access of Cancer Therapy across Europe; High Cost of Cancer Therapies.
8. Can you provide examples of recent developments in the market?
In May 2022 Boehringer Ingelheim acquired Northern Biologics, a wholly owned subsidiary of Northern LP. This acquisition has assisted the company to add two complementary assets to its existing cancer immunology portfolio including cancer vaccines, oncolytic viruses, T-cell engagers, and myeloid cell modulators.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Cancer Biological Therapy Industry in Europe," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Cancer Biological Therapy Industry in Europe report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Cancer Biological Therapy Industry in Europe?
To stay informed about further developments, trends, and reports in the Cancer Biological Therapy Industry in Europe, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
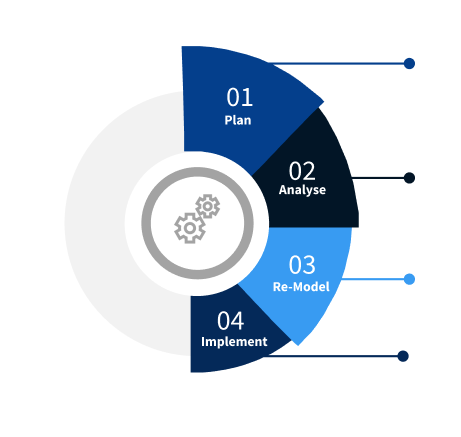
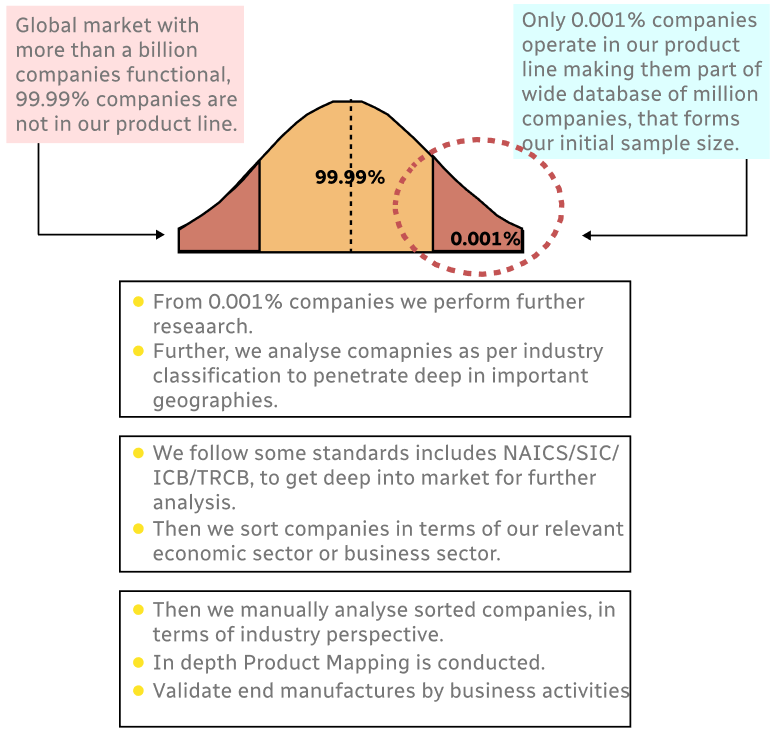
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


