Key Insights
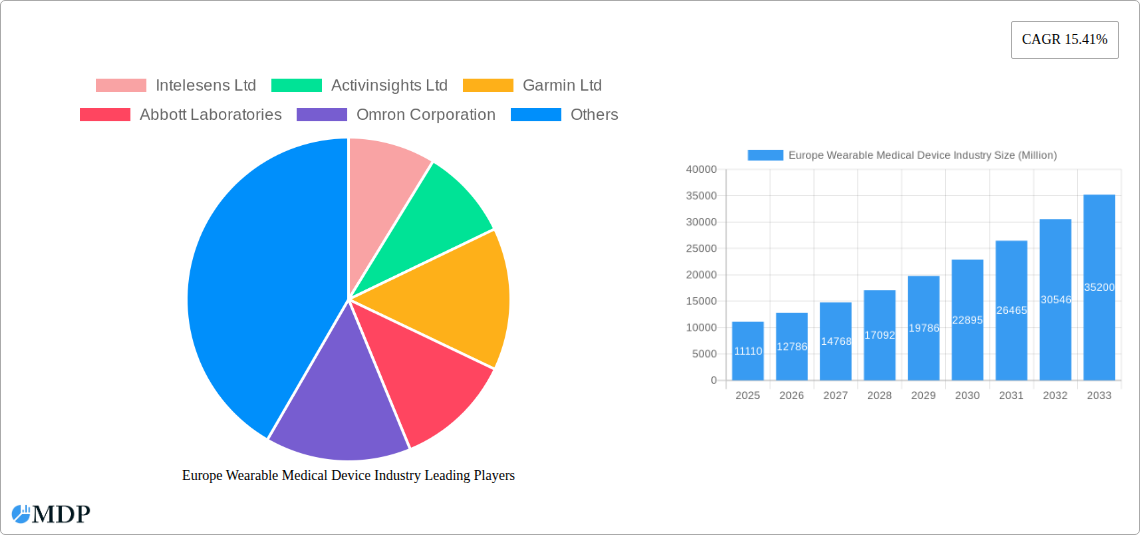
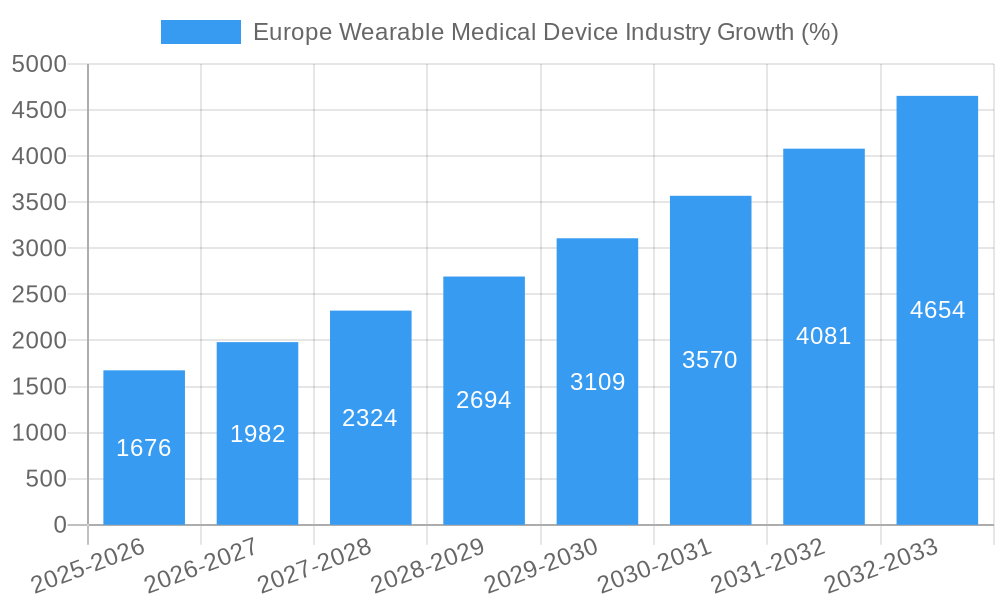
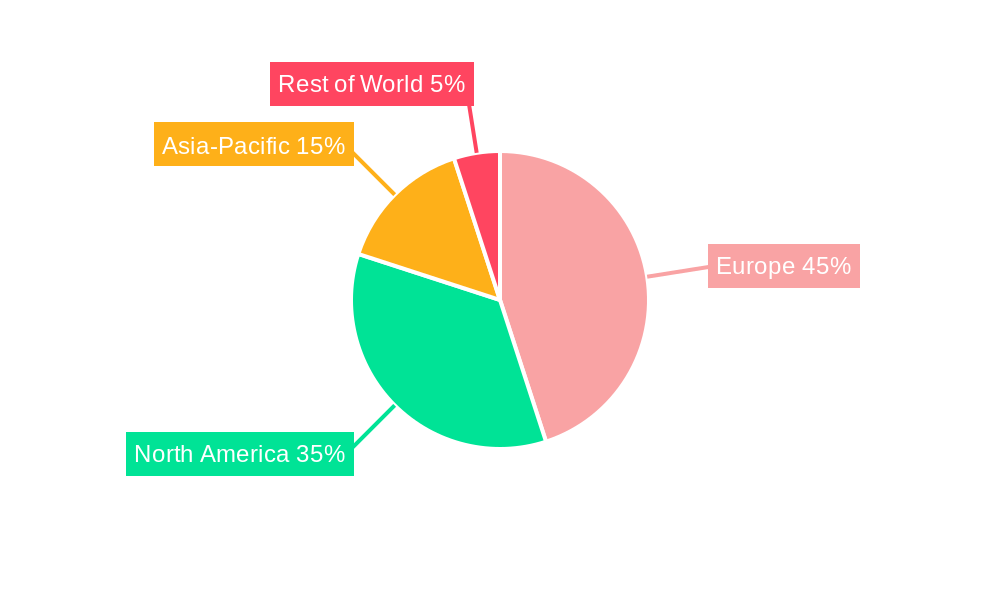
The European wearable medical device market, valued at €11.11 billion in 2025, is projected to experience robust growth, exhibiting a Compound Annual Growth Rate (CAGR) of 15.41% from 2025 to 2033. This expansion is driven by several key factors. The increasing prevalence of chronic diseases like diabetes and cardiovascular conditions necessitates continuous health monitoring, fueling demand for wearable devices offering remote patient monitoring (RPM) capabilities. Furthermore, the rising adoption of fitness trackers and smartwatches, coupled with advancements in sensor technology and data analytics, contribute significantly to market growth. Technological innovations enabling more accurate and convenient health data collection, along with the increasing affordability of wearable devices, are further accelerating market penetration. Key segments within the market include watches, wristbands, and ear wear, with monitoring devices and therapeutic neuromonitoring devices dominating the device type segment. The sports and fitness application segment currently holds a substantial market share, although the home healthcare and remote patient monitoring sectors are rapidly gaining traction, driven by the aging population and the increasing preference for convenient healthcare solutions. Germany, the United Kingdom, France, Italy, and Spain represent the largest national markets within Europe, collectively driving a significant portion of the regional growth.
Competition in the European wearable medical device market is intense, with established players like Garmin, Abbott Laboratories, Omron, and Philips alongside specialized companies such as Intelesens and Activinsights. These companies are continuously innovating to enhance product features, improve data accuracy, and develop integrated solutions that cater to the evolving needs of healthcare providers and consumers. Future growth will likely be influenced by factors such as regulatory approvals, data privacy concerns, and the integration of artificial intelligence (AI) and machine learning (ML) for advanced data analysis and personalized health management. The market's continued expansion relies on overcoming challenges such as ensuring data security and user privacy, addressing interoperability issues between different devices and healthcare systems, and promoting widespread adoption among diverse user demographics. Despite these challenges, the long-term outlook for the European wearable medical device market remains highly positive, driven by the aforementioned technological advancements and the growing awareness of preventative healthcare.
Europe Wearable Medical Device Industry: A Comprehensive Market Report (2019-2033)
This comprehensive report provides a detailed analysis of the European wearable medical device industry, offering invaluable insights for stakeholders seeking to understand market dynamics, growth opportunities, and competitive landscapes. With a study period spanning 2019-2033, a base year of 2025, and an estimated and forecast period of 2025-2033, this report covers historical data (2019-2024) and projects future trends. The report value is xx Million.
Europe Wearable Medical Device Industry Market Dynamics & Concentration
This section delves into the intricate dynamics shaping the European wearable medical device market. We analyze market concentration, identifying key players and their market share, exploring innovation drivers such as advancements in sensor technology and miniaturization, and examining the impact of the regulatory framework (e.g., GDPR, MDR) on market growth. The influence of product substitutes (e.g., traditional medical devices) and evolving end-user trends (e.g., increasing health consciousness, aging population) are also assessed. Finally, we analyze mergers and acquisitions (M&A) activity within the industry, quantifying deal counts and their impact on market consolidation.
- Market Concentration: The European wearable medical device market exhibits a moderately concentrated landscape, with a few major players holding significant market share. The exact figures will vary by segment and are detailed within the full report. We project a xx% market share for the top 5 players in 2025.
- Innovation Drivers: Miniaturization of sensors, improved data analytics capabilities, and the integration of AI are key innovation drivers.
- Regulatory Framework: The stringent regulatory environment in Europe, particularly concerning data privacy and medical device approvals, influences market entry and product development strategies. Compliance with MDR is a significant factor.
- Product Substitutes: Traditional medical devices pose a competitive threat, though the convenience and accessibility of wearables are proving increasingly attractive to consumers.
- End-User Trends: The rising prevalence of chronic diseases and the growing adoption of remote patient monitoring solutions are driving market demand.
- M&A Activity: The number of M&A deals in the sector has increased in recent years, reflecting consolidation efforts and the pursuit of technological advancements. The full report details a projected xx M&A deals for 2025–2033.
Europe Wearable Medical Device Industry Industry Trends & Analysis
This section provides a comprehensive overview of industry trends shaping the European wearable medical device market. We analyze the Compound Annual Growth Rate (CAGR) and market penetration of wearable medical devices across various segments. Factors driving market growth are examined including increasing healthcare expenditure, technological advancements, and changing consumer preferences. Furthermore, the competitive landscape and technological disruptions (e.g., advancements in AI and machine learning) are investigated.
The market exhibits a strong growth trajectory driven by factors such as the increasing prevalence of chronic diseases, rising healthcare expenditure, and advancements in technology. The CAGR is projected to be xx% during the forecast period (2025-2033). Market penetration is expected to increase significantly, particularly in segments like remote patient monitoring and home healthcare. Competitive dynamics are intense, with established players and new entrants vying for market share through product innovation and strategic partnerships.
Leading Markets & Segments in Europe Wearable Medical Device Industry
This section identifies the leading markets and segments within the European wearable medical device industry. Dominant regions, countries, and product/device types are analyzed, considering various factors that influence market leadership.
By Product Type:
- Watches: Watches represent a significant segment due to their versatility and established consumer base.
- Wristbands: Wristbands remain popular due to affordability and ease of use.
- Ear Wear: This segment is experiencing rapid growth driven by the increasing popularity of hearables.
- Other Product Types: This category includes diverse devices such as patches, clothing, and implants.
By Device Type:
- Monitoring Devices: This segment dominates the market due to the widespread adoption of fitness trackers and health monitoring devices.
- Neuromonitoring Devices: This segment is experiencing rapid growth driven by advancements in brain-computer interfaces and other neurotechnology.
- Therapeutic Devices: This segment is expected to witness substantial growth owing to the increasing demand for wearable drug delivery systems and other therapeutic applications.
By Application:
- Sports and Fitness: This remains a significant application, driven by the popularity of fitness tracking and wearable technology in sports and athletic activities.
- Remote Patient Monitoring: This segment is experiencing rapid growth due to its potential to improve healthcare access and reduce healthcare costs.
- Home Healthcare: This segment is growing due to the increasing need for convenient and cost-effective healthcare solutions at home.
Key drivers influencing segment dominance include economic policies supporting digital health initiatives, technological advancements, and evolving consumer preferences. Specific country-level analyses are presented in the full report. Germany and the UK are projected to be leading markets in 2025.
Europe Wearable Medical Device Industry Product Developments
Recent product innovations have focused on enhancing functionality, improving user experience, and expanding application areas. Miniaturization, improved sensor accuracy, and integration of advanced analytics are key technological trends. The market is witnessing a shift towards multi-functional devices that offer a broader range of health monitoring capabilities. For example, advancements in biosensors and AI are enabling more sophisticated and accurate health data collection and analysis. This allows for personalized interventions and preventative healthcare.
Key Drivers of Europe Wearable Medical Device Industry Growth
Several factors contribute to the growth of the European wearable medical device market. Technological advancements, such as miniaturized sensors and improved data processing capabilities, are crucial. The increasing prevalence of chronic diseases is another significant driver, as wearable devices provide convenient remote monitoring solutions. Favorable government regulations and funding initiatives supporting digital health also stimulate market growth. Furthermore, growing consumer awareness of health and wellness fuels demand for these devices.
Challenges in the Europe Wearable Medical Device Industry Market
Several challenges impede the growth of the European wearable medical device market. Stringent regulatory requirements and the associated costs of obtaining approvals represent major barriers. Concerns surrounding data privacy and security also pose a challenge. Furthermore, the competitive landscape, with established players and new entrants vying for market share, creates intense competition. Supply chain disruptions can also impact production and availability. These challenges influence market development and necessitate effective strategies to overcome these obstacles.
Emerging Opportunities in Europe Wearable Medical Device Industry
Significant opportunities for growth exist in the European wearable medical device market. Advancements in materials science and nanotechnology are paving the way for more comfortable and effective wearable devices. Strategic partnerships between device manufacturers and healthcare providers can create integrated solutions that improve patient care. Market expansion into underserved areas, such as remote regions and elderly populations, presents substantial opportunities for growth. The increasing focus on preventative healthcare is further fostering demand for wearable medical devices for early disease detection and management.
Leading Players in the Europe Wearable Medical Device Industry Sector
- Intelesens Ltd
- Activinsights Ltd
- Garmin Ltd
- Abbott Laboratories
- Omron Corporation
- Polar Electro Oy
- Fitbit Inc
- Nuubo
- Koninklijke Philips NV
Key Milestones in Europe Wearable Medical Device Industry Industry
March 2022: Infineon Technologies AG and Sleepiz AG launched Infineon XENSIV 60 GHz radar technology for contactless vital sign monitoring in smart home and healthcare devices. This technological advancement significantly impacts the market by enabling more convenient and accurate health monitoring.
February 2022: Oppo launched the Oppo Watch Free smartwatch in the European market. This launch enhanced the competitive landscape by offering a feature-rich fitness tracker at a potentially competitive price point. The increased sports tracking features, SpO2 sensor, and heart rate monitoring capabilities expanded the range of consumer options.
Strategic Outlook for Europe Wearable Medical Device Industry Market
The future of the European wearable medical device market is promising. Continued technological advancements, coupled with increasing consumer demand for personalized healthcare solutions, will drive significant growth. Strategic partnerships, focused on data integration and service delivery, will become increasingly crucial. Manufacturers who prioritize data privacy and security while embracing innovative technologies will gain a competitive edge. The market's long-term potential is substantial, driven by the confluence of technological innovation, evolving healthcare needs, and a supportive regulatory environment.
Europe Wearable Medical Device Industry Segmentation
-
1. Device Type
-
1.1. Monitoring Devices
- 1.1.1. Vital Sign Monitoring Devices
- 1.1.2. Sleep Monitoring Devices
- 1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 1.1.4. Neuromonitoring Devices
-
1.2. Therapeutic Devices
- 1.2.1. Pain Management Devices
- 1.2.2. Rehabilitation Devices
- 1.2.3. Respiratory Therapy Devices
- 1.2.4. Other Theraputic Devices
-
1.1. Monitoring Devices
-
2. Application
- 2.1. Sports and Fitness
- 2.2. Remote Patient Monitoring
- 2.3. Home Healthcare
-
3. Product Type
- 3.1. Watch
- 3.2. Wristband
- 3.3. Ear Wear
- 3.4. Other Product Types
Europe Wearable Medical Device Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Wearable Medical Device Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 15.41% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Technological Advancements and Innovation; Increasing Health Awareness; Ease of Use and Interpretation of Data
- 3.3. Market Restrains
- 3.3.1. Lack of Reimbursement Policies
- 3.4. Market Trends
- 3.4.1. Remote Patient Monitoring Segment is Expected to Grow Rapidly Over the Forecast Period in the Europe Wearable Medical Devices Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Monitoring Devices
- 5.1.1.1. Vital Sign Monitoring Devices
- 5.1.1.2. Sleep Monitoring Devices
- 5.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 5.1.1.4. Neuromonitoring Devices
- 5.1.2. Therapeutic Devices
- 5.1.2.1. Pain Management Devices
- 5.1.2.2. Rehabilitation Devices
- 5.1.2.3. Respiratory Therapy Devices
- 5.1.2.4. Other Theraputic Devices
- 5.1.1. Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Sports and Fitness
- 5.2.2. Remote Patient Monitoring
- 5.2.3. Home Healthcare
- 5.3. Market Analysis, Insights and Forecast - by Product Type
- 5.3.1. Watch
- 5.3.2. Wristband
- 5.3.3. Ear Wear
- 5.3.4. Other Product Types
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. Germany
- 5.4.2. United Kingdom
- 5.4.3. France
- 5.4.4. Italy
- 5.4.5. Spain
- 5.4.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Germany Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Monitoring Devices
- 6.1.1.1. Vital Sign Monitoring Devices
- 6.1.1.2. Sleep Monitoring Devices
- 6.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 6.1.1.4. Neuromonitoring Devices
- 6.1.2. Therapeutic Devices
- 6.1.2.1. Pain Management Devices
- 6.1.2.2. Rehabilitation Devices
- 6.1.2.3. Respiratory Therapy Devices
- 6.1.2.4. Other Theraputic Devices
- 6.1.1. Monitoring Devices
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Sports and Fitness
- 6.2.2. Remote Patient Monitoring
- 6.2.3. Home Healthcare
- 6.3. Market Analysis, Insights and Forecast - by Product Type
- 6.3.1. Watch
- 6.3.2. Wristband
- 6.3.3. Ear Wear
- 6.3.4. Other Product Types
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. United Kingdom Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Monitoring Devices
- 7.1.1.1. Vital Sign Monitoring Devices
- 7.1.1.2. Sleep Monitoring Devices
- 7.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 7.1.1.4. Neuromonitoring Devices
- 7.1.2. Therapeutic Devices
- 7.1.2.1. Pain Management Devices
- 7.1.2.2. Rehabilitation Devices
- 7.1.2.3. Respiratory Therapy Devices
- 7.1.2.4. Other Theraputic Devices
- 7.1.1. Monitoring Devices
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Sports and Fitness
- 7.2.2. Remote Patient Monitoring
- 7.2.3. Home Healthcare
- 7.3. Market Analysis, Insights and Forecast - by Product Type
- 7.3.1. Watch
- 7.3.2. Wristband
- 7.3.3. Ear Wear
- 7.3.4. Other Product Types
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. France Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Monitoring Devices
- 8.1.1.1. Vital Sign Monitoring Devices
- 8.1.1.2. Sleep Monitoring Devices
- 8.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 8.1.1.4. Neuromonitoring Devices
- 8.1.2. Therapeutic Devices
- 8.1.2.1. Pain Management Devices
- 8.1.2.2. Rehabilitation Devices
- 8.1.2.3. Respiratory Therapy Devices
- 8.1.2.4. Other Theraputic Devices
- 8.1.1. Monitoring Devices
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Sports and Fitness
- 8.2.2. Remote Patient Monitoring
- 8.2.3. Home Healthcare
- 8.3. Market Analysis, Insights and Forecast - by Product Type
- 8.3.1. Watch
- 8.3.2. Wristband
- 8.3.3. Ear Wear
- 8.3.4. Other Product Types
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. Italy Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Monitoring Devices
- 9.1.1.1. Vital Sign Monitoring Devices
- 9.1.1.2. Sleep Monitoring Devices
- 9.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 9.1.1.4. Neuromonitoring Devices
- 9.1.2. Therapeutic Devices
- 9.1.2.1. Pain Management Devices
- 9.1.2.2. Rehabilitation Devices
- 9.1.2.3. Respiratory Therapy Devices
- 9.1.2.4. Other Theraputic Devices
- 9.1.1. Monitoring Devices
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Sports and Fitness
- 9.2.2. Remote Patient Monitoring
- 9.2.3. Home Healthcare
- 9.3. Market Analysis, Insights and Forecast - by Product Type
- 9.3.1. Watch
- 9.3.2. Wristband
- 9.3.3. Ear Wear
- 9.3.4. Other Product Types
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. Spain Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Monitoring Devices
- 10.1.1.1. Vital Sign Monitoring Devices
- 10.1.1.2. Sleep Monitoring Devices
- 10.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 10.1.1.4. Neuromonitoring Devices
- 10.1.2. Therapeutic Devices
- 10.1.2.1. Pain Management Devices
- 10.1.2.2. Rehabilitation Devices
- 10.1.2.3. Respiratory Therapy Devices
- 10.1.2.4. Other Theraputic Devices
- 10.1.1. Monitoring Devices
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Sports and Fitness
- 10.2.2. Remote Patient Monitoring
- 10.2.3. Home Healthcare
- 10.3. Market Analysis, Insights and Forecast - by Product Type
- 10.3.1. Watch
- 10.3.2. Wristband
- 10.3.3. Ear Wear
- 10.3.4. Other Product Types
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. Rest of Europe Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 11.1.1. Monitoring Devices
- 11.1.1.1. Vital Sign Monitoring Devices
- 11.1.1.2. Sleep Monitoring Devices
- 11.1.1.3. Electrocardiographs, Fetal and Obstetric Devices
- 11.1.1.4. Neuromonitoring Devices
- 11.1.2. Therapeutic Devices
- 11.1.2.1. Pain Management Devices
- 11.1.2.2. Rehabilitation Devices
- 11.1.2.3. Respiratory Therapy Devices
- 11.1.2.4. Other Theraputic Devices
- 11.1.1. Monitoring Devices
- 11.2. Market Analysis, Insights and Forecast - by Application
- 11.2.1. Sports and Fitness
- 11.2.2. Remote Patient Monitoring
- 11.2.3. Home Healthcare
- 11.3. Market Analysis, Insights and Forecast - by Product Type
- 11.3.1. Watch
- 11.3.2. Wristband
- 11.3.3. Ear Wear
- 11.3.4. Other Product Types
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 12. Germany Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 13. United Kingdom Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 14. France Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 15. Italy Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 16. Spain Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 17. Rest of Europe Europe Wearable Medical Device Industry Analysis, Insights and Forecast, 2019-2031
- 18. Competitive Analysis
- 18.1. Market Share Analysis 2024
- 18.2. Company Profiles
- 18.2.1 Intelesens Ltd
- 18.2.1.1. Overview
- 18.2.1.2. Products
- 18.2.1.3. SWOT Analysis
- 18.2.1.4. Recent Developments
- 18.2.1.5. Financials (Based on Availability)
- 18.2.2 Activinsights Ltd
- 18.2.2.1. Overview
- 18.2.2.2. Products
- 18.2.2.3. SWOT Analysis
- 18.2.2.4. Recent Developments
- 18.2.2.5. Financials (Based on Availability)
- 18.2.3 Garmin Ltd
- 18.2.3.1. Overview
- 18.2.3.2. Products
- 18.2.3.3. SWOT Analysis
- 18.2.3.4. Recent Developments
- 18.2.3.5. Financials (Based on Availability)
- 18.2.4 Abbott Laboratories
- 18.2.4.1. Overview
- 18.2.4.2. Products
- 18.2.4.3. SWOT Analysis
- 18.2.4.4. Recent Developments
- 18.2.4.5. Financials (Based on Availability)
- 18.2.5 Omron Corporation
- 18.2.5.1. Overview
- 18.2.5.2. Products
- 18.2.5.3. SWOT Analysis
- 18.2.5.4. Recent Developments
- 18.2.5.5. Financials (Based on Availability)
- 18.2.6 Polar Electro Oy*List Not Exhaustive
- 18.2.6.1. Overview
- 18.2.6.2. Products
- 18.2.6.3. SWOT Analysis
- 18.2.6.4. Recent Developments
- 18.2.6.5. Financials (Based on Availability)
- 18.2.7 Fitbit Inc
- 18.2.7.1. Overview
- 18.2.7.2. Products
- 18.2.7.3. SWOT Analysis
- 18.2.7.4. Recent Developments
- 18.2.7.5. Financials (Based on Availability)
- 18.2.8 Nuubo
- 18.2.8.1. Overview
- 18.2.8.2. Products
- 18.2.8.3. SWOT Analysis
- 18.2.8.4. Recent Developments
- 18.2.8.5. Financials (Based on Availability)
- 18.2.9 Koninklinje Philips NV
- 18.2.9.1. Overview
- 18.2.9.2. Products
- 18.2.9.3. SWOT Analysis
- 18.2.9.4. Recent Developments
- 18.2.9.5. Financials (Based on Availability)
- 18.2.1 Intelesens Ltd
List of Figures
- Figure 1: Europe Wearable Medical Device Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Wearable Medical Device Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Wearable Medical Device Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Wearable Medical Device Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 3: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 4: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 5: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 6: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 7: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 8: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 9: Europe Wearable Medical Device Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 10: Europe Wearable Medical Device Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 11: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 12: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 13: Germany Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Germany Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 15: United Kingdom Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: United Kingdom Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 17: France Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: France Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 19: Italy Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Italy Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 21: Spain Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Spain Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 23: Rest of Europe Europe Wearable Medical Device Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: Rest of Europe Europe Wearable Medical Device Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 25: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 26: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 27: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 28: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 29: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 30: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 31: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 32: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 33: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 34: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 35: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 36: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 37: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 38: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 39: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 40: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 41: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 42: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 43: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 44: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 45: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 46: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 47: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 48: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 49: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 50: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 51: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 52: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 53: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 54: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 55: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 56: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 57: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 58: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 59: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 60: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 61: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 62: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 63: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 64: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 65: Europe Wearable Medical Device Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 66: Europe Wearable Medical Device Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 67: Europe Wearable Medical Device Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 68: Europe Wearable Medical Device Industry Volume K Units Forecast, by Application 2019 & 2032
- Table 69: Europe Wearable Medical Device Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 70: Europe Wearable Medical Device Industry Volume K Units Forecast, by Product Type 2019 & 2032
- Table 71: Europe Wearable Medical Device Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 72: Europe Wearable Medical Device Industry Volume K Units Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Wearable Medical Device Industry?
The projected CAGR is approximately 15.41%.
2. Which companies are prominent players in the Europe Wearable Medical Device Industry?
Key companies in the market include Intelesens Ltd, Activinsights Ltd, Garmin Ltd, Abbott Laboratories, Omron Corporation, Polar Electro Oy*List Not Exhaustive, Fitbit Inc, Nuubo, Koninklinje Philips NV.
3. What are the main segments of the Europe Wearable Medical Device Industry?
The market segments include Device Type, Application, Product Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 11.11 Million as of 2022.
5. What are some drivers contributing to market growth?
Technological Advancements and Innovation; Increasing Health Awareness; Ease of Use and Interpretation of Data.
6. What are the notable trends driving market growth?
Remote Patient Monitoring Segment is Expected to Grow Rapidly Over the Forecast Period in the Europe Wearable Medical Devices Market.
7. Are there any restraints impacting market growth?
Lack of Reimbursement Policies.
8. Can you provide examples of recent developments in the market?
IN March 2022, Infineon Technologies AG in collaboration with Sleepiz AG launched Infineon XENSIV 60 GHz radar technology, integrated into smart home and healthcare devices, that offers a great opportunity for healthcare applications as they allow to accurately measure vital signs such as heartbeat and breathing rate without touching the body.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Wearable Medical Device Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Wearable Medical Device Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Wearable Medical Device Industry?
To stay informed about further developments, trends, and reports in the Europe Wearable Medical Device Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


