Key Insights
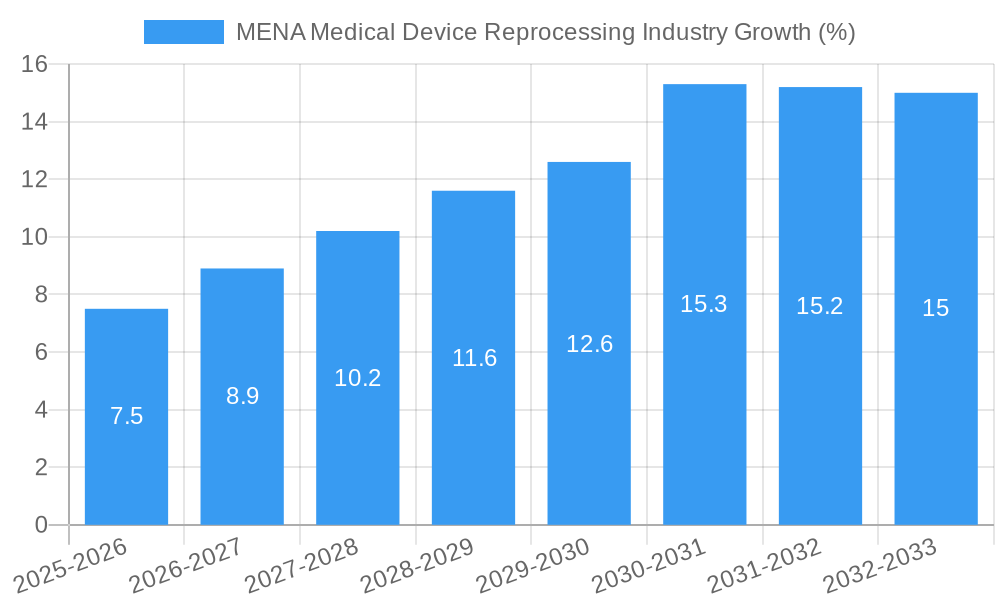
The MENA (Middle East and North Africa) medical device reprocessing industry is experiencing robust growth, driven by increasing healthcare expenditure, a rising prevalence of chronic diseases, and a growing emphasis on cost-effectiveness within healthcare systems. The market's expansion is further fueled by stringent regulations promoting infection control and the adoption of advanced reprocessing technologies. While precise figures for the MENA region are not explicitly provided, the global CAGR of 15.93% suggests significant potential for this regional market. Considering the expanding healthcare infrastructure and increasing population in several MENA countries, a conservative estimate for the 2025 MENA market size would be around $50 million, with a projected growth trajectory exceeding the global average due to factors specific to the region, such as increasing public-private partnerships in healthcare. This growth is likely to be further bolstered by government initiatives aimed at improving healthcare infrastructure and access, thus increasing demand for efficient and reliable medical device reprocessing services.
The market segmentation reveals a dominance of Class I devices, which are often simpler to reprocess, contributing to the higher volume of reprocessing services. However, the increasing use of sophisticated Class II devices, with their more complex reprocessing requirements, presents opportunities for specialized service providers. Key players such as Arjo, Johnson & Johnson, and Stryker are likely to play a significant role in shaping this market, driving technological innovation and potentially through mergers and acquisitions. The regional distribution of this market is likely uneven, with countries like the UAE and Saudi Arabia experiencing faster growth due to their advanced healthcare systems and higher per capita healthcare spending. Challenges such as a lack of skilled professionals and inconsistencies in regulatory frameworks across different MENA nations might hinder the market's full potential. However, investments in training and standardization initiatives are expected to address these challenges over the forecast period (2025-2033).
MENA Medical Device Reprocessing Industry Report: 2019-2033
Unlocking Growth in a Thriving Market: A Comprehensive Analysis of the MENA Medical Device Reprocessing Sector
This comprehensive report provides an in-depth analysis of the Medical Device Reprocessing industry in the Middle East and North Africa (MENA) region, covering the period from 2019 to 2033. We delve into market dynamics, leading players, emerging trends, and future opportunities, equipping stakeholders with actionable insights to navigate this rapidly evolving landscape. With a focus on key segments like Class I and Class II devices, this report is essential for investors, manufacturers, healthcare providers, and regulatory bodies seeking a clear understanding of the MENA medical device reprocessing market. The report features a detailed analysis of market size, reaching xx Million by 2025 and projected to reach xx Million by 2033, showcasing a robust CAGR of xx%.
MENA Medical Device Reprocessing Industry Market Dynamics & Concentration
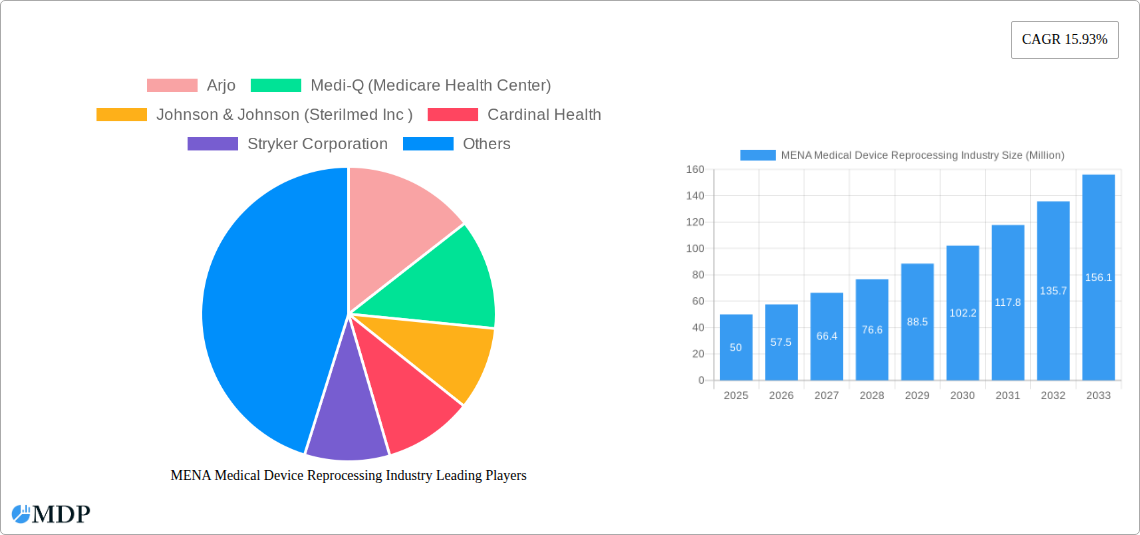
The MENA medical device reprocessing market is characterized by a moderate level of concentration, with a few major players holding significant market share. However, the market exhibits substantial growth potential driven by increasing healthcare expenditure, rising prevalence of chronic diseases, and the growing adoption of advanced medical technologies. Stringent regulatory frameworks are shaping market practices, encouraging the adoption of standardized reprocessing techniques. The presence of substitute technologies, while limited, adds a layer of competitive pressure. End-user trends favor automated and efficient reprocessing solutions, driving innovation in the sector. Mergers and acquisitions (M&A) activity within the MENA region remains relatively low, with an estimated xx M&A deals recorded between 2019 and 2024. Key players such as Arjo, Johnson & Johnson (Sterilmed Inc), Cardinal Health, Stryker Corporation, and Getinge AB are actively shaping market dynamics through product innovation and strategic partnerships. Market share distribution among these key players is estimated at approximately xx% for the top three players, indicating room for expansion by emerging companies and specialized niche players.
MENA Medical Device Reprocessing Industry Industry Trends & Analysis
The MENA medical device reprocessing market is experiencing robust growth, driven by several key factors. The increasing prevalence of infectious diseases and the consequent need for stringent infection control measures are fueling demand for effective reprocessing solutions. Furthermore, technological advancements in automation, sterilization techniques, and monitoring systems are enhancing the efficiency and safety of reprocessing procedures. This is reflected in the market's strong CAGR of xx% during the forecast period (2025-2033). Consumer preferences are shifting towards advanced, automated systems that minimize human intervention and improve the accuracy of the reprocessing cycle. Market penetration of automated reprocessing systems is projected to reach xx% by 2033, driven by substantial investments in healthcare infrastructure and increasing awareness of infection control protocols. The competitive landscape is characterized by a mix of established global players and regional companies, leading to increased innovation and price competition.
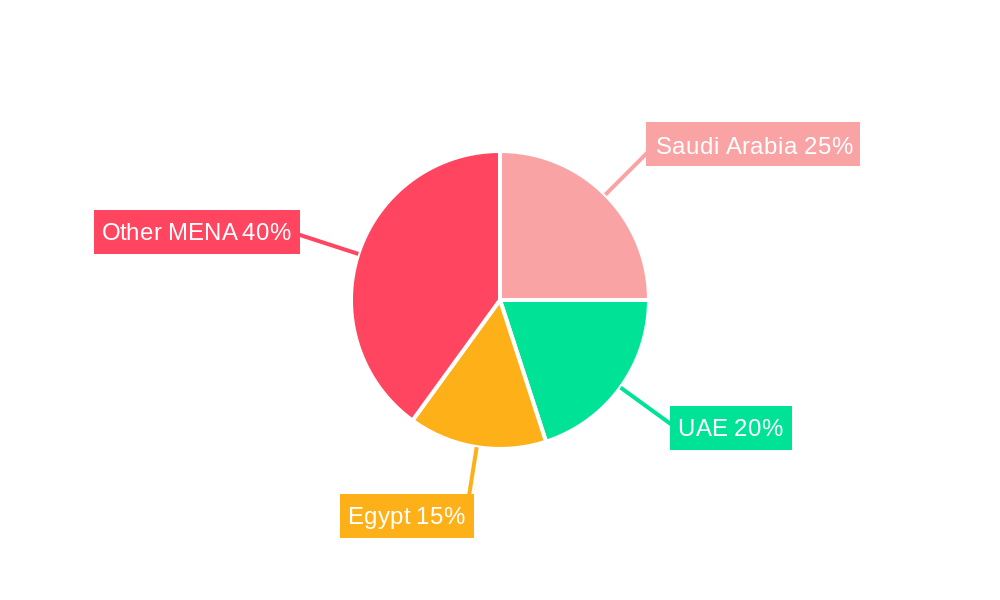
Leading Markets & Segments in MENA Medical Device Reprocessing Industry
The UAE and Saudi Arabia are currently the leading markets in the MENA medical device reprocessing sector, driven by robust healthcare infrastructure, higher healthcare spending per capita, and increased adoption of advanced medical technologies.
- Key Drivers in the UAE and Saudi Arabia:
- Significant investments in healthcare infrastructure and modernization initiatives.
- Government support for healthcare technology adoption and infection control programs.
- A growing private sector healthcare landscape and increasing medical tourism.
- Stringent regulatory frameworks promoting high-quality reprocessing standards.
The Class II devices segment dominates the market, fueled by the high demand for advanced medical equipment across hospitals and surgical centers. Class I devices maintain a steady market share, especially in primary healthcare facilities and smaller clinics. This dominance is attributable to:
- Higher complexity and specialized nature of Class II devices compared to Class I devices.
- Higher adoption rates of Class II devices in advanced medical procedures.
- Technological advancements driving innovation in Class II devices.
MENA Medical Device Reprocessing Industry Product Developments
Recent product innovations have focused on enhancing automation, efficiency, and traceability in medical device reprocessing. Automated endoscope reprocessors and advanced sterilization technologies are gaining traction, improving the safety and reliability of the process. These innovations address critical market needs for increased efficiency, reduced manual handling, and improved documentation and tracking capabilities. The emphasis on improved tracking and data management systems is streamlining reprocessing workflows and improving compliance with regulatory requirements.
Key Drivers of MENA Medical Device Reprocessing Industry Growth
The growth of the MENA medical device reprocessing market is fueled by a confluence of technological, economic, and regulatory factors. Technological advancements, such as automated reprocessing systems, are increasing efficiency and safety. Economic factors, such as rising healthcare expenditure and the growing private healthcare sector, are driving demand. Furthermore, supportive government policies and regulatory frameworks promoting infection control standards are boosting market growth. The increasing prevalence of chronic diseases and the rising number of surgical procedures are further propelling market expansion.
Challenges in the MENA Medical Device Reprocessing Industry Market
The MENA medical device reprocessing market faces challenges, including the high cost of advanced technologies, especially automated reprocessing systems, creating a barrier for smaller facilities. Supply chain disruptions, potentially affecting the availability of critical supplies and equipment, pose a significant risk. Furthermore, varying regulatory landscapes across different MENA countries can create compliance complexities. Competitive pressures from both established international players and regional companies intensify market competition. These challenges negatively impact market expansion and profitability of individual players.
Emerging Opportunities in MENA Medical Device Reprocessing Industry
The MENA medical device reprocessing market presents significant long-term growth opportunities. Technological breakthroughs in automation, artificial intelligence, and sterilization techniques are poised to reshape the industry. Strategic partnerships between manufacturers and healthcare providers can foster innovation and improve market penetration. Expansion into underserved markets and leveraging digital health solutions for remote monitoring and data management create further opportunities for significant growth and market expansion over the forecast period.
Leading Players in the MENA Medical Device Reprocessing Industry Sector
- Arjo
- Medi-Q (Medicare Health Center)
- Johnson & Johnson (Sterilmed Inc)
- Cardinal Health
- Stryker Corporation
- NEScientific Inc
- Getinge AB
- Medline Industries Inc
Key Milestones in MENA Medical Device Reprocessing Industry Industry
- June 2022: The Ministry of Health (MOH) in Oman launched a workshop on endoscope reprocessing in collaboration with the WHO, showcasing state-of-the-art equipment and techniques, raising awareness and setting higher standards.
- March 2022: Getinge AB established a training center in Dubai, enhancing market access and training on advanced reprocessing solutions, thereby boosting technology adoption and enhancing market penetration.
Strategic Outlook for MENA Medical Device Reprocessing Industry Market
The MENA medical device reprocessing market holds significant future potential, driven by technological innovation, increasing healthcare investment, and supportive regulatory frameworks. Strategic partnerships, focusing on technology transfer and capacity building, will be crucial for sustaining growth. Companies that effectively address regulatory compliance, supply chain resilience, and patient safety concerns will be well-positioned to capitalize on the expanding market opportunities. The focus on automation, data-driven solutions, and enhanced traceability will be pivotal for long-term success within this dynamic sector.
MENA Medical Device Reprocessing Industry Segmentation
-
1. Device Type
-
1.1. Class I Devices
- 1.1.1. Laparoscopic Graspers
- 1.1.2. Scissors
- 1.1.3. Forceps
- 1.1.4. Scalpels
- 1.1.5. Tourniquet Cuffs
- 1.1.6. Other Class I Devices
-
1.2. Class II Devices
- 1.2.1. Pulse Oximeter Sensors
- 1.2.2. Sequential Compression Sleeves
- 1.2.3. Catheters and Guidewires
- 1.2.4. Other Class II Devices
-
1.1. Class I Devices
-
2. Geography
-
2.1. Middle East and Africa
- 2.1.1. GCC
- 2.1.2. South Africa
- 2.1.3. Rest of Middle East and Africa
-
2.1. Middle East and Africa
MENA Medical Device Reprocessing Industry Segmentation By Geography
- 1. North America: United States Canada Mexico
- 2. Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe
- 3. Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific
- 4. South America : Brazil, Argentina, Rest of South America
- 5. MEA: Middle East, Africa
MENA Medical Device Reprocessing Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 15.93% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Cost Savings Through Reprocessing Single-use Devices; Rising Need to Reduce the Volume of Medical Waste
- 3.3. Market Restrains
- 3.3.1. Potential for Material Alteration and Cross Infection with Reprocessed Devices; Preconceived Notions Regarding the Quality of Reprocessed Single-use Medical Devices (SUDs); Lack of Regulations for Reprocessing in the Middle East and Africa
- 3.4. Market Trends
- 3.4.1. Catheters and Guidewires in the Class II Devices Segment are Anticipated to Grow Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Class I Devices
- 5.1.1.1. Laparoscopic Graspers
- 5.1.1.2. Scissors
- 5.1.1.3. Forceps
- 5.1.1.4. Scalpels
- 5.1.1.5. Tourniquet Cuffs
- 5.1.1.6. Other Class I Devices
- 5.1.2. Class II Devices
- 5.1.2.1. Pulse Oximeter Sensors
- 5.1.2.2. Sequential Compression Sleeves
- 5.1.2.3. Catheters and Guidewires
- 5.1.2.4. Other Class II Devices
- 5.1.1. Class I Devices
- 5.2. Market Analysis, Insights and Forecast - by Geography
- 5.2.1. Middle East and Africa
- 5.2.1.1. GCC
- 5.2.1.2. South Africa
- 5.2.1.3. Rest of Middle East and Africa
- 5.2.1. Middle East and Africa
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America: United States Canada Mexico
- 5.3.2. Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe
- 5.3.3. Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific
- 5.3.4. South America : Brazil, Argentina, Rest of South America
- 5.3.5. MEA: Middle East, Africa
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Class I Devices
- 6.1.1.1. Laparoscopic Graspers
- 6.1.1.2. Scissors
- 6.1.1.3. Forceps
- 6.1.1.4. Scalpels
- 6.1.1.5. Tourniquet Cuffs
- 6.1.1.6. Other Class I Devices
- 6.1.2. Class II Devices
- 6.1.2.1. Pulse Oximeter Sensors
- 6.1.2.2. Sequential Compression Sleeves
- 6.1.2.3. Catheters and Guidewires
- 6.1.2.4. Other Class II Devices
- 6.1.1. Class I Devices
- 6.2. Market Analysis, Insights and Forecast - by Geography
- 6.2.1. Middle East and Africa
- 6.2.1.1. GCC
- 6.2.1.2. South Africa
- 6.2.1.3. Rest of Middle East and Africa
- 6.2.1. Middle East and Africa
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Class I Devices
- 7.1.1.1. Laparoscopic Graspers
- 7.1.1.2. Scissors
- 7.1.1.3. Forceps
- 7.1.1.4. Scalpels
- 7.1.1.5. Tourniquet Cuffs
- 7.1.1.6. Other Class I Devices
- 7.1.2. Class II Devices
- 7.1.2.1. Pulse Oximeter Sensors
- 7.1.2.2. Sequential Compression Sleeves
- 7.1.2.3. Catheters and Guidewires
- 7.1.2.4. Other Class II Devices
- 7.1.1. Class I Devices
- 7.2. Market Analysis, Insights and Forecast - by Geography
- 7.2.1. Middle East and Africa
- 7.2.1.1. GCC
- 7.2.1.2. South Africa
- 7.2.1.3. Rest of Middle East and Africa
- 7.2.1. Middle East and Africa
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Class I Devices
- 8.1.1.1. Laparoscopic Graspers
- 8.1.1.2. Scissors
- 8.1.1.3. Forceps
- 8.1.1.4. Scalpels
- 8.1.1.5. Tourniquet Cuffs
- 8.1.1.6. Other Class I Devices
- 8.1.2. Class II Devices
- 8.1.2.1. Pulse Oximeter Sensors
- 8.1.2.2. Sequential Compression Sleeves
- 8.1.2.3. Catheters and Guidewires
- 8.1.2.4. Other Class II Devices
- 8.1.1. Class I Devices
- 8.2. Market Analysis, Insights and Forecast - by Geography
- 8.2.1. Middle East and Africa
- 8.2.1.1. GCC
- 8.2.1.2. South Africa
- 8.2.1.3. Rest of Middle East and Africa
- 8.2.1. Middle East and Africa
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Class I Devices
- 9.1.1.1. Laparoscopic Graspers
- 9.1.1.2. Scissors
- 9.1.1.3. Forceps
- 9.1.1.4. Scalpels
- 9.1.1.5. Tourniquet Cuffs
- 9.1.1.6. Other Class I Devices
- 9.1.2. Class II Devices
- 9.1.2.1. Pulse Oximeter Sensors
- 9.1.2.2. Sequential Compression Sleeves
- 9.1.2.3. Catheters and Guidewires
- 9.1.2.4. Other Class II Devices
- 9.1.1. Class I Devices
- 9.2. Market Analysis, Insights and Forecast - by Geography
- 9.2.1. Middle East and Africa
- 9.2.1.1. GCC
- 9.2.1.2. South Africa
- 9.2.1.3. Rest of Middle East and Africa
- 9.2.1. Middle East and Africa
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Class I Devices
- 10.1.1.1. Laparoscopic Graspers
- 10.1.1.2. Scissors
- 10.1.1.3. Forceps
- 10.1.1.4. Scalpels
- 10.1.1.5. Tourniquet Cuffs
- 10.1.1.6. Other Class I Devices
- 10.1.2. Class II Devices
- 10.1.2.1. Pulse Oximeter Sensors
- 10.1.2.2. Sequential Compression Sleeves
- 10.1.2.3. Catheters and Guidewires
- 10.1.2.4. Other Class II Devices
- 10.1.1. Class I Devices
- 10.2. Market Analysis, Insights and Forecast - by Geography
- 10.2.1. Middle East and Africa
- 10.2.1.1. GCC
- 10.2.1.2. South Africa
- 10.2.1.3. Rest of Middle East and Africa
- 10.2.1. Middle East and Africa
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. North America MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 11.1.1 United States
- 11.1.2 Canada
- 11.1.3 Mexico
- 12. Europe MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 12.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 12.1.1 Germany
- 12.1.2 United Kingdom
- 12.1.3 France
- 12.1.4 Spain
- 12.1.5 Italy
- 12.1.6 Spain
- 12.1.7 Belgium
- 12.1.8 Netherland
- 12.1.9 Nordics
- 12.1.10 Rest of Europe
- 13. Asia Pacific MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 13.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 13.1.1 China
- 13.1.2 Japan
- 13.1.3 India
- 13.1.4 South Korea
- 13.1.5 Southeast Asia
- 13.1.6 Australia
- 13.1.7 Indonesia
- 13.1.8 Phillipes
- 13.1.9 Singapore
- 13.1.10 Thailandc
- 13.1.11 Rest of Asia Pacific
- 14. South America MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 14.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 14.1.1 Brazil
- 14.1.2 Argentina
- 14.1.3 Peru
- 14.1.4 Chile
- 14.1.5 Colombia
- 14.1.6 Ecuador
- 14.1.7 Venezuela
- 14.1.8 Rest of South America
- 15. North America MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 15.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 15.1.1 United States
- 15.1.2 Canada
- 15.1.3 Mexico
- 16. MEA MENA Medical Device Reprocessing Industry Analysis, Insights and Forecast, 2019-2031
- 16.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 16.1.1 United Arab Emirates
- 16.1.2 Saudi Arabia
- 16.1.3 South Africa
- 16.1.4 Rest of Middle East and Africa
- 17. Competitive Analysis
- 17.1. Global Market Share Analysis 2024
- 17.2. Company Profiles
- 17.2.1 Arjo
- 17.2.1.1. Overview
- 17.2.1.2. Products
- 17.2.1.3. SWOT Analysis
- 17.2.1.4. Recent Developments
- 17.2.1.5. Financials (Based on Availability)
- 17.2.2 Medi-Q (Medicare Health Center)
- 17.2.2.1. Overview
- 17.2.2.2. Products
- 17.2.2.3. SWOT Analysis
- 17.2.2.4. Recent Developments
- 17.2.2.5. Financials (Based on Availability)
- 17.2.3 Johnson & Johnson (Sterilmed Inc )
- 17.2.3.1. Overview
- 17.2.3.2. Products
- 17.2.3.3. SWOT Analysis
- 17.2.3.4. Recent Developments
- 17.2.3.5. Financials (Based on Availability)
- 17.2.4 Cardinal Health
- 17.2.4.1. Overview
- 17.2.4.2. Products
- 17.2.4.3. SWOT Analysis
- 17.2.4.4. Recent Developments
- 17.2.4.5. Financials (Based on Availability)
- 17.2.5 Stryker Corporation
- 17.2.5.1. Overview
- 17.2.5.2. Products
- 17.2.5.3. SWOT Analysis
- 17.2.5.4. Recent Developments
- 17.2.5.5. Financials (Based on Availability)
- 17.2.6 NEScientific Inc
- 17.2.6.1. Overview
- 17.2.6.2. Products
- 17.2.6.3. SWOT Analysis
- 17.2.6.4. Recent Developments
- 17.2.6.5. Financials (Based on Availability)
- 17.2.7 Getinge AB
- 17.2.7.1. Overview
- 17.2.7.2. Products
- 17.2.7.3. SWOT Analysis
- 17.2.7.4. Recent Developments
- 17.2.7.5. Financials (Based on Availability)
- 17.2.8 Medline Industries Inc
- 17.2.8.1. Overview
- 17.2.8.2. Products
- 17.2.8.3. SWOT Analysis
- 17.2.8.4. Recent Developments
- 17.2.8.5. Financials (Based on Availability)
- 17.2.1 Arjo
List of Figures
- Figure 1: Global MENA Medical Device Reprocessing Industry Revenue Breakdown (Million, %) by Region 2024 & 2032
- Figure 2: North America MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 3: North America MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 4: Europe MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 5: Europe MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 6: Asia Pacific MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 7: Asia Pacific MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 9: South America MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 10: North America MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 11: North America MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 12: MEA MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 13: MEA MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 14: North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 15: North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 16: North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Revenue (Million), by Geography 2024 & 2032
- Figure 17: North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Revenue Share (%), by Geography 2024 & 2032
- Figure 18: North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 19: North America: United States Canada Mexico MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 20: Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 21: Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 22: Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Revenue (Million), by Geography 2024 & 2032
- Figure 23: Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Revenue Share (%), by Geography 2024 & 2032
- Figure 24: Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 25: Europe: Germany: France: Italy: United Kingdom Netherlands Rest of Europe MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 27: Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 28: Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Revenue (Million), by Geography 2024 & 2032
- Figure 29: Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Revenue Share (%), by Geography 2024 & 2032
- Figure 30: Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 31: Asia Pacific: China, Japan, India, South Korea, Taiwan, Australia, Rest of Asia-Pacific MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 32: South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 33: South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 34: South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Revenue (Million), by Geography 2024 & 2032
- Figure 35: South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Revenue Share (%), by Geography 2024 & 2032
- Figure 36: South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 37: South America : Brazil, Argentina, Rest of South America MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
- Figure 38: MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 39: MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 40: MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Revenue (Million), by Geography 2024 & 2032
- Figure 41: MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Revenue Share (%), by Geography 2024 & 2032
- Figure 42: MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Revenue (Million), by Country 2024 & 2032
- Figure 43: MEA: Middle East, Africa MENA Medical Device Reprocessing Industry Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 3: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 4: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 5: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 6: United States MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Canada MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Mexico MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 10: Germany MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: United Kingdom MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: France MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Spain MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Italy MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 15: Spain MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Belgium MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 17: Netherland MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Nordics MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 19: Rest of Europe MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 21: China MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Japan MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 23: India MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: South Korea MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 25: Southeast Asia MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 26: Australia MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 27: Indonesia MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: Phillipes MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 29: Singapore MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: Thailandc MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 31: Rest of Asia Pacific MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 32: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 33: Brazil MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Argentina MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 35: Peru MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 36: Chile MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 37: Colombia MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: Ecuador MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 39: Venezuela MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 40: Rest of South America MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 41: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 42: United States MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 43: Canada MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 44: Mexico MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 45: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 46: United Arab Emirates MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 47: Saudi Arabia MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 48: South Africa MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 49: Rest of Middle East and Africa MENA Medical Device Reprocessing Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 50: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 51: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 52: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 53: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 54: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 55: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 56: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 57: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 58: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 59: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 60: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 61: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 62: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 63: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 64: Global MENA Medical Device Reprocessing Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the MENA Medical Device Reprocessing Industry?
The projected CAGR is approximately 15.93%.
2. Which companies are prominent players in the MENA Medical Device Reprocessing Industry?
Key companies in the market include Arjo, Medi-Q (Medicare Health Center), Johnson & Johnson (Sterilmed Inc ), Cardinal Health, Stryker Corporation, NEScientific Inc, Getinge AB, Medline Industries Inc.
3. What are the main segments of the MENA Medical Device Reprocessing Industry?
The market segments include Device Type, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 579.65 Million as of 2022.
5. What are some drivers contributing to market growth?
Cost Savings Through Reprocessing Single-use Devices; Rising Need to Reduce the Volume of Medical Waste.
6. What are the notable trends driving market growth?
Catheters and Guidewires in the Class II Devices Segment are Anticipated to Grow Over the Forecast Period.
7. Are there any restraints impacting market growth?
Potential for Material Alteration and Cross Infection with Reprocessed Devices; Preconceived Notions Regarding the Quality of Reprocessed Single-use Medical Devices (SUDs); Lack of Regulations for Reprocessing in the Middle East and Africa.
8. Can you provide examples of recent developments in the market?
June 2022: The Ministry of Health (MOH) launched a workshop in Oman on endoscope reprocessing in collaboration with WHO. The event involved an exhibition displaying state-of-the-art equipment and techniques for medical device reprocessing.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "MENA Medical Device Reprocessing Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the MENA Medical Device Reprocessing Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the MENA Medical Device Reprocessing Industry?
To stay informed about further developments, trends, and reports in the MENA Medical Device Reprocessing Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


