Key Insights
The European vascular access device market, valued at approximately €[Estimate based on market size XX and exchange rate. For example, if XX = $100 million and the €/$ exchange rate is 0.9, the value would be €90 million] in 2025, is projected to experience steady growth, driven by a 2.50% CAGR from 2025 to 2033. This growth is fueled by several key factors. The increasing prevalence of chronic diseases such as diabetes and cancer, requiring long-term vascular access for medication delivery and dialysis, significantly boosts demand. Technological advancements leading to the development of less invasive, longer-lasting, and safer devices also contribute to market expansion. Furthermore, the rising geriatric population in Europe, prone to conditions necessitating vascular access, further fuels market growth. The increasing adoption of minimally invasive surgical techniques within hospitals and clinics is another significant driver. However, the market faces challenges, including stringent regulatory approvals and the potential for complications associated with vascular access devices. These challenges necessitate ongoing research and development of safer and more effective devices to ensure market sustainability.
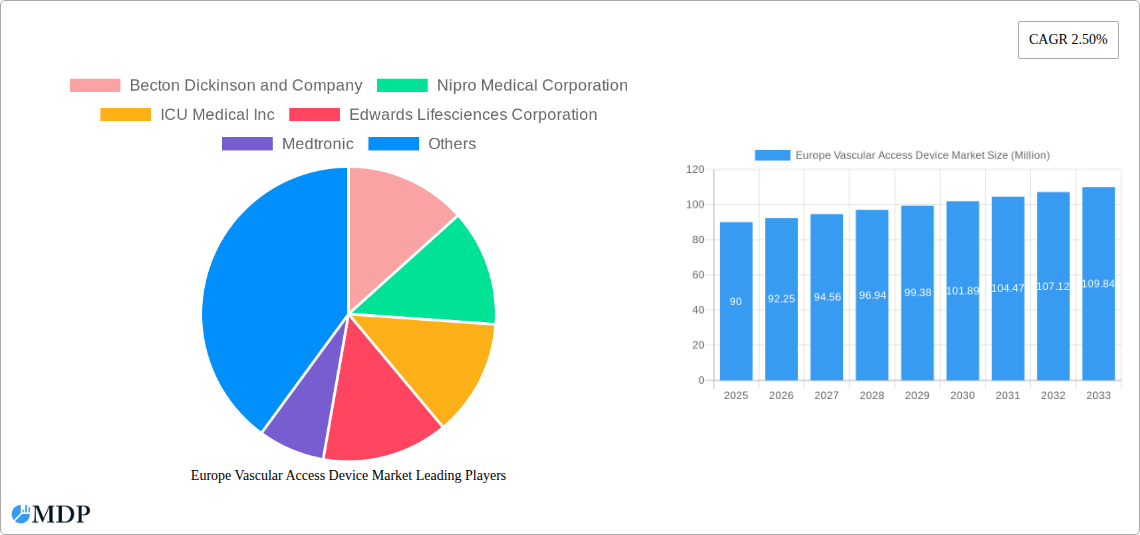
The European market is segmented by device type (central and peripheral vascular access devices), application (drug administration, fluid and nutrition administration, diagnostics, and other applications), and end-user (hospitals/clinics, diagnostic centers, and other end-users). Germany, France, Italy, the United Kingdom, and other key European nations contribute significantly to the market’s overall value. The competitive landscape is characterized by established players like Becton Dickinson, Nipro, ICU Medical, Edwards Lifesciences, Medtronic, and Fresenius Medical Care, alongside several other significant companies constantly innovating to maintain market share. The market's future trajectory hinges on the continued development of innovative devices, coupled with successful navigation of regulatory hurdles and cost-effectiveness considerations within the healthcare systems of various European countries. The market presents opportunities for companies focusing on advanced materials, improved device design, and enhanced patient safety features.
Europe Vascular Access Device Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the Europe Vascular Access Device Market, offering valuable insights for stakeholders across the medical device industry. The study covers the period 2019-2033, with a focus on the 2025-2033 forecast period. The market is segmented by device type, application, and end-user, providing a granular understanding of its diverse landscape. Key players such as Becton Dickinson and Company, Nipro Medical Corporation, ICU Medical Inc, Edwards Lifesciences Corporation, Medtronic, Fresenius Medical Care AG & Co KGaA, 3M, AngioDynamics, Terumo Medical Corporation, Teleflex Incorporated, B Braun SE, and Prodimed are analyzed, along with emerging trends and opportunities. The report projects a market value of xx Million by 2033, showcasing substantial growth potential. Download now to gain a competitive edge!
Europe Vascular Access Device Market Market Dynamics & Concentration
The European vascular access device market exhibits a moderately concentrated structure, with several major players holding significant market share. However, the presence of numerous smaller companies and ongoing innovation fosters a dynamic competitive landscape. The market is driven by factors such as the rising prevalence of chronic diseases requiring frequent vascular access, technological advancements leading to improved device designs and functionalities, and supportive regulatory frameworks promoting innovation. Product substitutes, primarily less invasive procedures, pose some challenges. However, the advantages of vascular access devices in terms of efficacy and versatility limit their impact. End-user trends indicate a shift towards minimally invasive procedures and a growing preference for devices offering enhanced patient comfort and safety. M&A activities in the sector are moderate, with xx deals recorded between 2019 and 2024, contributing to market consolidation and technological integration.
- Market Concentration: Moderately concentrated, with top 5 players holding approximately xx% market share in 2024.
- Innovation Drivers: Miniaturization, improved biocompatibility, and smart features.
- Regulatory Landscape: Primarily compliant with EU medical device regulations (MDR).
- Product Substitutes: Minimally invasive procedures and alternative therapies.
- End-User Trends: Preference for minimally invasive, comfortable, and safe devices.
- M&A Activity: xx mergers and acquisitions between 2019 and 2024.
Europe Vascular Access Device Market Industry Trends & Analysis
The Europe vascular access device market is projected to experience robust growth, with a CAGR of xx% during the forecast period (2025-2033). This growth is fueled by several key factors. The aging population across Europe contributes to a higher incidence of chronic diseases requiring frequent vascular access. Technological advancements, such as the development of implantable devices and improved catheter materials, are enhancing device efficacy and patient comfort. Increasing healthcare expenditure and a rising focus on patient-centric care further contribute to market expansion. However, the market faces challenges including price pressure, stringent regulatory requirements, and potential supply chain disruptions. Competitive dynamics are characterized by both fierce competition among established players and the emergence of innovative startups. Market penetration of advanced devices remains relatively low, indicating significant growth potential. Consumer preferences are increasingly focused on safety, efficacy, and ease of use.
Leading Markets & Segments in Europe Vascular Access Device Market
The German market currently holds the leading position within Europe, driven by robust healthcare infrastructure and high prevalence of chronic diseases. Other key markets include France, the UK, Italy, and Spain.
By Device Type:
- Central Vascular Access Devices: Holds the largest market share due to its extensive use in various applications. Growth is driven by demand for long-term access solutions.
- Peripheral Vascular Access Devices: Experiencing moderate growth due to the increasing use in outpatient settings and improved designs.
By Application:
- Administration of Drugs: Represents the dominant application segment, driven by the rising prevalence of chronic diseases requiring frequent drug administration.
- Administration of Fluid and Nutrition: A significant segment, particularly in critical care settings.
By End-User:
- Hospital/Clinic: The largest end-user segment, attributable to the high volume of procedures performed in hospitals and clinics.
- Diagnostic Centers: A growing segment, driven by the increasing demand for diagnostic procedures requiring vascular access.
Key Drivers:
- High prevalence of chronic diseases requiring vascular access
- Advancements in device technology
- Increased healthcare expenditure
- Favorable regulatory environment (in specific countries)
Europe Vascular Access Device Market Product Developments
Recent product innovations focus on enhancing safety, minimizing complications, and improving patient comfort. This includes the development of biocompatible materials, minimally invasive insertion techniques, and smart devices with improved monitoring capabilities. These advancements are driving market growth by addressing unmet needs and improving treatment outcomes. The market is witnessing a gradual shift towards minimally invasive and implantable devices, reflecting the increasing demand for convenient and long-term solutions. The integration of smart technologies, such as sensors and data analytics, offers significant potential to improve patient care and outcomes.
Key Drivers of Europe Vascular Access Device Market Growth
The European vascular access device market is experiencing robust growth driven by several key factors: the increasing prevalence of chronic diseases such as cancer and diabetes necessitating frequent intravenous therapies; technological advancements resulting in safer, more efficient, and user-friendly devices; the rising geriatric population demanding longer-term vascular access solutions; and supportive government regulations fostering innovation in the medical device sector. Furthermore, substantial investments in healthcare infrastructure across several European nations are augmenting market growth.
Challenges in the Europe Vascular Access Device Market Market
Despite the market's positive growth trajectory, several challenges hinder its progress. Stringent regulatory approval processes can lead to extended product launch timelines, increasing costs. Supply chain vulnerabilities pose risks to the availability of essential raw materials and components, potentially affecting production capacities. Intense competition among numerous established players and emerging companies exerts pricing pressure, impacting profitability. These factors create an environment that requires manufacturers to balance innovation with cost-effectiveness to maintain a competitive edge. The approximate impact of these challenges on the overall market growth is estimated to be around xx%.
Emerging Opportunities in Europe Vascular Access Device Market
The European vascular access device market presents considerable opportunities for long-term growth. Technological breakthroughs such as the development of biocompatible, implantable devices with enhanced longevity and sophisticated monitoring capabilities present significant growth potential. Strategic partnerships between established companies and innovative startups offer synergies that drive product development and market penetration. Expansion into underserved regions of Europe through targeted marketing and distribution strategies presents another growth pathway. Furthermore, the increasing demand for home healthcare solutions is expanding the market's reach beyond traditional hospital settings.
Leading Players in the Europe Vascular Access Device Market Sector
- Becton Dickinson and Company
- Nipro Medical Corporation
- ICU Medical Inc
- Edwards Lifesciences Corporation
- Medtronic
- Fresenius Medical Care AG & Co KGaA
- 3M
- AngioDynamics
- Terumo Medical Corporation
- Teleflex Incorporated
- B Braun SE
- Prodimed
Key Milestones in Europe Vascular Access Device Market Industry
- June 2023: BIOTRONIK launched its Oscar multifunctional peripheral catheter, expanding treatment options for peripheral artery disease. This launch enhances competitive dynamics by introducing a new advanced device.
- September 2022: Delta Med Group's strategic partnership with Pentaferte broadens market reach and competition in the infusion medical device sector. This signifies increased collaboration and market consolidation.
Strategic Outlook for Europe Vascular Access Device Market Market
The future of the European vascular access device market is bright, driven by a confluence of factors. Continued technological advancements promise safer, more effective devices, boosting market demand. Strategic collaborations and mergers and acquisitions will further consolidate the industry, leading to enhanced efficiency and innovation. Focus on patient-centric care and a rising emphasis on minimally invasive procedures will reshape the market. The increasing prevalence of chronic diseases and an aging population guarantee sustained growth potential. The market presents numerous opportunities for companies with robust innovation capabilities and strategic vision.
Europe Vascular Access Device Market Segmentation
-
1. Device Type
-
1.1. Central Vascular Access Devices
- 1.1.1. Peripherally Inserted Central Catheters
- 1.1.2. Percutaneous Non-tunneled Catheters
- 1.1.3. Other Central Vascular Access Devices
-
1.2. Peripheral Vascular Access Devices
- 1.2.1. Peripheral Catheter
- 1.2.2. Midline Catheter
- 1.2.3. Other Peripheral Vascular Access Devices
-
1.1. Central Vascular Access Devices
-
2. Application
- 2.1. Administration of Drugs
- 2.2. Administration of Fluid and Nutrition
- 2.3. Diagnostics and Testing
- 2.4. Other Applications
-
3. End-User
- 3.1. Hospital/Clinic
- 3.2. Diagnostic Centers
- 3.3. Other End-Users
Europe Vascular Access Device Market Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Vascular Access Device Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 2.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Growing prevalence of Chronic Disease; Increasing Number of Chemotherapy Procedures with High Hospitalization Rates; Rising Use of Vascular Access Devices among Paediatric Patients
- 3.3. Market Restrains
- 3.3.1. Risks Associated with Catheter Usage; Stringent Regulations and Product Recalls
- 3.4. Market Trends
- 3.4.1. The Administration of Drugs Segment is Expected to Garner Significant Share of the Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Central Vascular Access Devices
- 5.1.1.1. Peripherally Inserted Central Catheters
- 5.1.1.2. Percutaneous Non-tunneled Catheters
- 5.1.1.3. Other Central Vascular Access Devices
- 5.1.2. Peripheral Vascular Access Devices
- 5.1.2.1. Peripheral Catheter
- 5.1.2.2. Midline Catheter
- 5.1.2.3. Other Peripheral Vascular Access Devices
- 5.1.1. Central Vascular Access Devices
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Administration of Drugs
- 5.2.2. Administration of Fluid and Nutrition
- 5.2.3. Diagnostics and Testing
- 5.2.4. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by End-User
- 5.3.1. Hospital/Clinic
- 5.3.2. Diagnostic Centers
- 5.3.3. Other End-Users
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. Germany
- 5.4.2. United Kingdom
- 5.4.3. France
- 5.4.4. Italy
- 5.4.5. Spain
- 5.4.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Germany Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Central Vascular Access Devices
- 6.1.1.1. Peripherally Inserted Central Catheters
- 6.1.1.2. Percutaneous Non-tunneled Catheters
- 6.1.1.3. Other Central Vascular Access Devices
- 6.1.2. Peripheral Vascular Access Devices
- 6.1.2.1. Peripheral Catheter
- 6.1.2.2. Midline Catheter
- 6.1.2.3. Other Peripheral Vascular Access Devices
- 6.1.1. Central Vascular Access Devices
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Administration of Drugs
- 6.2.2. Administration of Fluid and Nutrition
- 6.2.3. Diagnostics and Testing
- 6.2.4. Other Applications
- 6.3. Market Analysis, Insights and Forecast - by End-User
- 6.3.1. Hospital/Clinic
- 6.3.2. Diagnostic Centers
- 6.3.3. Other End-Users
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. United Kingdom Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Central Vascular Access Devices
- 7.1.1.1. Peripherally Inserted Central Catheters
- 7.1.1.2. Percutaneous Non-tunneled Catheters
- 7.1.1.3. Other Central Vascular Access Devices
- 7.1.2. Peripheral Vascular Access Devices
- 7.1.2.1. Peripheral Catheter
- 7.1.2.2. Midline Catheter
- 7.1.2.3. Other Peripheral Vascular Access Devices
- 7.1.1. Central Vascular Access Devices
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Administration of Drugs
- 7.2.2. Administration of Fluid and Nutrition
- 7.2.3. Diagnostics and Testing
- 7.2.4. Other Applications
- 7.3. Market Analysis, Insights and Forecast - by End-User
- 7.3.1. Hospital/Clinic
- 7.3.2. Diagnostic Centers
- 7.3.3. Other End-Users
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. France Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Central Vascular Access Devices
- 8.1.1.1. Peripherally Inserted Central Catheters
- 8.1.1.2. Percutaneous Non-tunneled Catheters
- 8.1.1.3. Other Central Vascular Access Devices
- 8.1.2. Peripheral Vascular Access Devices
- 8.1.2.1. Peripheral Catheter
- 8.1.2.2. Midline Catheter
- 8.1.2.3. Other Peripheral Vascular Access Devices
- 8.1.1. Central Vascular Access Devices
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Administration of Drugs
- 8.2.2. Administration of Fluid and Nutrition
- 8.2.3. Diagnostics and Testing
- 8.2.4. Other Applications
- 8.3. Market Analysis, Insights and Forecast - by End-User
- 8.3.1. Hospital/Clinic
- 8.3.2. Diagnostic Centers
- 8.3.3. Other End-Users
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. Italy Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Central Vascular Access Devices
- 9.1.1.1. Peripherally Inserted Central Catheters
- 9.1.1.2. Percutaneous Non-tunneled Catheters
- 9.1.1.3. Other Central Vascular Access Devices
- 9.1.2. Peripheral Vascular Access Devices
- 9.1.2.1. Peripheral Catheter
- 9.1.2.2. Midline Catheter
- 9.1.2.3. Other Peripheral Vascular Access Devices
- 9.1.1. Central Vascular Access Devices
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Administration of Drugs
- 9.2.2. Administration of Fluid and Nutrition
- 9.2.3. Diagnostics and Testing
- 9.2.4. Other Applications
- 9.3. Market Analysis, Insights and Forecast - by End-User
- 9.3.1. Hospital/Clinic
- 9.3.2. Diagnostic Centers
- 9.3.3. Other End-Users
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. Spain Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Central Vascular Access Devices
- 10.1.1.1. Peripherally Inserted Central Catheters
- 10.1.1.2. Percutaneous Non-tunneled Catheters
- 10.1.1.3. Other Central Vascular Access Devices
- 10.1.2. Peripheral Vascular Access Devices
- 10.1.2.1. Peripheral Catheter
- 10.1.2.2. Midline Catheter
- 10.1.2.3. Other Peripheral Vascular Access Devices
- 10.1.1. Central Vascular Access Devices
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Administration of Drugs
- 10.2.2. Administration of Fluid and Nutrition
- 10.2.3. Diagnostics and Testing
- 10.2.4. Other Applications
- 10.3. Market Analysis, Insights and Forecast - by End-User
- 10.3.1. Hospital/Clinic
- 10.3.2. Diagnostic Centers
- 10.3.3. Other End-Users
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. Rest of Europe Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 11.1.1. Central Vascular Access Devices
- 11.1.1.1. Peripherally Inserted Central Catheters
- 11.1.1.2. Percutaneous Non-tunneled Catheters
- 11.1.1.3. Other Central Vascular Access Devices
- 11.1.2. Peripheral Vascular Access Devices
- 11.1.2.1. Peripheral Catheter
- 11.1.2.2. Midline Catheter
- 11.1.2.3. Other Peripheral Vascular Access Devices
- 11.1.1. Central Vascular Access Devices
- 11.2. Market Analysis, Insights and Forecast - by Application
- 11.2.1. Administration of Drugs
- 11.2.2. Administration of Fluid and Nutrition
- 11.2.3. Diagnostics and Testing
- 11.2.4. Other Applications
- 11.3. Market Analysis, Insights and Forecast - by End-User
- 11.3.1. Hospital/Clinic
- 11.3.2. Diagnostic Centers
- 11.3.3. Other End-Users
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 12. Germany Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 13. France Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 14. Italy Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 15. United Kingdom Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 16. Netherlands Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 17. Sweden Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 18. Rest of Europe Europe Vascular Access Device Market Analysis, Insights and Forecast, 2019-2031
- 19. Competitive Analysis
- 19.1. Market Share Analysis 2024
- 19.2. Company Profiles
- 19.2.1 Becton Dickinson and Company
- 19.2.1.1. Overview
- 19.2.1.2. Products
- 19.2.1.3. SWOT Analysis
- 19.2.1.4. Recent Developments
- 19.2.1.5. Financials (Based on Availability)
- 19.2.2 Nipro Medical Corporation
- 19.2.2.1. Overview
- 19.2.2.2. Products
- 19.2.2.3. SWOT Analysis
- 19.2.2.4. Recent Developments
- 19.2.2.5. Financials (Based on Availability)
- 19.2.3 ICU Medical Inc
- 19.2.3.1. Overview
- 19.2.3.2. Products
- 19.2.3.3. SWOT Analysis
- 19.2.3.4. Recent Developments
- 19.2.3.5. Financials (Based on Availability)
- 19.2.4 Edwards Lifesciences Corporation
- 19.2.4.1. Overview
- 19.2.4.2. Products
- 19.2.4.3. SWOT Analysis
- 19.2.4.4. Recent Developments
- 19.2.4.5. Financials (Based on Availability)
- 19.2.5 Medtronic
- 19.2.5.1. Overview
- 19.2.5.2. Products
- 19.2.5.3. SWOT Analysis
- 19.2.5.4. Recent Developments
- 19.2.5.5. Financials (Based on Availability)
- 19.2.6 Fresenius Medical Care AG & Co KGaA*List Not Exhaustive
- 19.2.6.1. Overview
- 19.2.6.2. Products
- 19.2.6.3. SWOT Analysis
- 19.2.6.4. Recent Developments
- 19.2.6.5. Financials (Based on Availability)
- 19.2.7 3M
- 19.2.7.1. Overview
- 19.2.7.2. Products
- 19.2.7.3. SWOT Analysis
- 19.2.7.4. Recent Developments
- 19.2.7.5. Financials (Based on Availability)
- 19.2.8 AngioDynamics
- 19.2.8.1. Overview
- 19.2.8.2. Products
- 19.2.8.3. SWOT Analysis
- 19.2.8.4. Recent Developments
- 19.2.8.5. Financials (Based on Availability)
- 19.2.9 Terumo Medical Corporation
- 19.2.9.1. Overview
- 19.2.9.2. Products
- 19.2.9.3. SWOT Analysis
- 19.2.9.4. Recent Developments
- 19.2.9.5. Financials (Based on Availability)
- 19.2.10 Teleflex Incorporated
- 19.2.10.1. Overview
- 19.2.10.2. Products
- 19.2.10.3. SWOT Analysis
- 19.2.10.4. Recent Developments
- 19.2.10.5. Financials (Based on Availability)
- 19.2.11 B Braun SE
- 19.2.11.1. Overview
- 19.2.11.2. Products
- 19.2.11.3. SWOT Analysis
- 19.2.11.4. Recent Developments
- 19.2.11.5. Financials (Based on Availability)
- 19.2.12 Prodimed
- 19.2.12.1. Overview
- 19.2.12.2. Products
- 19.2.12.3. SWOT Analysis
- 19.2.12.4. Recent Developments
- 19.2.12.5. Financials (Based on Availability)
- 19.2.1 Becton Dickinson and Company
List of Figures
- Figure 1: Europe Vascular Access Device Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Vascular Access Device Market Share (%) by Company 2024
List of Tables
- Table 1: Europe Vascular Access Device Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 3: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 4: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 5: Europe Vascular Access Device Market Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
- Table 7: Germany Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: France Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Italy Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: United Kingdom Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: Netherlands Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Sweden Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Rest of Europe Europe Vascular Access Device Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 15: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 16: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 17: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
- Table 18: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 19: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 20: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 21: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
- Table 22: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 23: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 24: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 25: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
- Table 26: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 27: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 28: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 29: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
- Table 30: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 31: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 32: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 33: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
- Table 34: Europe Vascular Access Device Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 35: Europe Vascular Access Device Market Revenue Million Forecast, by Application 2019 & 2032
- Table 36: Europe Vascular Access Device Market Revenue Million Forecast, by End-User 2019 & 2032
- Table 37: Europe Vascular Access Device Market Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Vascular Access Device Market?
The projected CAGR is approximately 2.50%.
2. Which companies are prominent players in the Europe Vascular Access Device Market?
Key companies in the market include Becton Dickinson and Company, Nipro Medical Corporation, ICU Medical Inc, Edwards Lifesciences Corporation, Medtronic, Fresenius Medical Care AG & Co KGaA*List Not Exhaustive, 3M, AngioDynamics, Terumo Medical Corporation, Teleflex Incorporated, B Braun SE, Prodimed.
3. What are the main segments of the Europe Vascular Access Device Market?
The market segments include Device Type, Application, End-User.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Growing prevalence of Chronic Disease; Increasing Number of Chemotherapy Procedures with High Hospitalization Rates; Rising Use of Vascular Access Devices among Paediatric Patients.
6. What are the notable trends driving market growth?
The Administration of Drugs Segment is Expected to Garner Significant Share of the Market.
7. Are there any restraints impacting market growth?
Risks Associated with Catheter Usage; Stringent Regulations and Product Recalls.
8. Can you provide examples of recent developments in the market?
June 2023: BIOTRONIK launched its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and started promotional activities at LINC, the Leipzig Interventional Course held in Leipzig, Germany. The device offers access into and to dilate stenoses in femoral, popliteal, and infrapopliteal arteries.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Vascular Access Device Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Vascular Access Device Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Vascular Access Device Market?
To stay informed about further developments, trends, and reports in the Europe Vascular Access Device Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


